Stromal cells maintain immune cell homeostasis in adipose tissue via production of interleukin-33
- PMID: 31053655
- PMCID: PMC6766755
- DOI: 10.1126/sciimmunol.aax0416
Stromal cells maintain immune cell homeostasis in adipose tissue via production of interleukin-33
Abstract
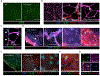
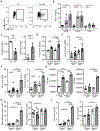
Obesity is driven by chronic low-grade inflammation resulting from dysregulated immune cell accumulation and function in white adipose tissue (WAT). Interleukin-33 (IL-33) is a key cytokine that controls innate and adaptive immune cell activity and immune homeostasis in WAT, although the sources of IL-33 have remained controversial. Here, we show that WAT-resident mesenchyme-derived stromal cells are the dominant producers of IL-33. Adipose stem and progenitor cells (ASPCs) produced IL-33 in all WAT depots, whereas mesothelial cells served as an additional source of IL-33 in visceral WAT. ASPC-derived IL-33 promoted a regulatory circuit that maintained an immune tone in WAT via the induction of group 2 innate lymphoid cell-derived type 2 cytokines and maintenance of eosinophils, whereas mesothelial IL-33 also acted as an alarmin by inducing peritoneal immune response upon infection. Together, these data reveal a previously unrecognized regulatory network between tissue-resident progenitor cells and innate lymphoid cells that maintains immune homeostasis in adipose tissue.
Copyright © 2019 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures




Comment in
-
Stromal IL-33 balances fat stores.Nat Rev Immunol. 2019 Jul;19(7):412-413. doi: 10.1038/s41577-019-0179-7. Nat Rev Immunol. 2019. PMID: 31110269 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

