Structural basis of βTrCP1-associated GLI3 processing
- PMID: 31053742
- PMCID: PMC6499770
- DOI: 10.1038/s41598-019-43392-3
Structural basis of βTrCP1-associated GLI3 processing
Abstract
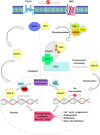
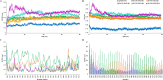
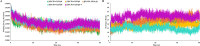
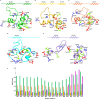
Controlled ubiquitin-mediated protein degradation is essential for various cellular processes. GLI family regulates the transcriptional events of the sonic hedgehog pathway genes that are implicated in almost one fourth of human tumors. GLI3 phosphorylation by Ser/Thr kinases is a primary factor for their transcriptional activity that incurs the formation of both GLI3 repressor and activator forms. GLI3 processing is triggered in an ubiquitin-dependent manner via SCFβTrCP1 complex; however, structural characterization, mode of action based on sequence of phosphorylation signatures and induced conformational readjustments remain elusive. Here, through structural analysis and molecular dynamics simulation assays, we explored comparative binding pattern of GLI3 phosphopeptides against βTrCP1. A comprehensive and thorough analysis demarcated GLI3 presence in the binding cleft shared by inter-bladed binding grooves of β-propeller. Our results revealed the involvement of all seven WD40 repeats of βTrCP1 in GLI3 interaction. Conversely, GLI3 phosphorylation pattern at primary protein kinase A (PKA) sites and secondary casein kinase 1 (CK1) or glycogen synthase kinase 3 (GSK3) sites was carefully evaluated. Our results indicated that GLI3 processing depends on the 19 phosphorylation sites (849, 852, 855, 856, 860, 861, 864, 865, 868, 872, 873, 876, 877, 880, 899, 903, 906, 907 and 910 positions) by a cascade of PKA, GSK3β and CSKI kinases. The presence of a sequential phosphorylation in the binding induction of GLI3 and βTrCP1 may be a hallmark to authenticate GLI3 processing. We speculate that mechanistic information of the individual residual contributions through structure-guided approaches may be pivotal for the rational design of specific and more potent inhibitors against activated GLI3 with a special emphasis on the anticancer activity.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Antiviral drug acyclovir exhibits antitumor activity via targeting βTrCP1: Molecular docking and dynamics simulation study.J Mol Graph Model. 2017 Mar;72:96-105. doi: 10.1016/j.jmgm.2016.12.018. Epub 2016 Dec 31. J Mol Graph Model. 2017. PMID: 28092836
-
Multisite protein kinase A and glycogen synthase kinase 3beta phosphorylation leads to Gli3 ubiquitination by SCFbetaTrCP.Mol Cell Biol. 2006 Jun;26(11):4316-26. doi: 10.1128/MCB.02183-05. Mol Cell Biol. 2006. PMID: 16705181 Free PMC article.
-
Sonic hedgehog signaling regulates Gli2 transcriptional activity by suppressing its processing and degradation.Mol Cell Biol. 2006 May;26(9):3365-77. doi: 10.1128/MCB.26.9.3365-3377.2006. Mol Cell Biol. 2006. PMID: 16611981 Free PMC article.
-
Elucidation, functional clustering and structural characterization of βTrCP1 substrates through a molecular dynamics study.Mol Biosyst. 2016 Jun 21;12(7):2233-46. doi: 10.1039/c6mb00189k. Mol Biosyst. 2016. PMID: 27156994
-
Evidence for the direct involvement of {beta}TrCP in Gli3 protein processing.Proc Natl Acad Sci U S A. 2006 Jan 3;103(1):33-8. doi: 10.1073/pnas.0509927103. Epub 2005 Dec 21. Proc Natl Acad Sci U S A. 2006. PMID: 16371461 Free PMC article.
Cited by
-
E2UbcH5B-derived peptide ligands target HECT E3-E2 binding site and block the Ub-dependent SARS-CoV-2 egression: A computational study.Comput Biol Med. 2022 Jul;146:105660. doi: 10.1016/j.compbiomed.2022.105660. Epub 2022 May 22. Comput Biol Med. 2022. PMID: 35751189 Free PMC article.
-
Defining the Role of GLI/Hedgehog Signaling in Chemoresistance: Implications in Therapeutic Approaches.Cancers (Basel). 2021 Sep 23;13(19):4746. doi: 10.3390/cancers13194746. Cancers (Basel). 2021. PMID: 34638233 Free PMC article. Review.
-
Oncogenic potential of truncated-Gli3 via the Gsk3β/Gli3/AR-V7 axis in castration-resistant prostate cancer.Oncogene. 2025 Apr;44(15):1007-1023. doi: 10.1038/s41388-024-03266-z. Epub 2025 Jan 16. Oncogene. 2025. PMID: 39821099 Free PMC article.
-
The Role of Smoothened-Dependent and -Independent Hedgehog Signaling Pathway in Tumorigenesis.Biomedicines. 2021 Sep 10;9(9):1188. doi: 10.3390/biomedicines9091188. Biomedicines. 2021. PMID: 34572373 Free PMC article. Review.
-
Counting Degrons: Lessons From Multivalent Substrates for Targeted Protein Degradation.Front Physiol. 2022 Jul 4;13:913063. doi: 10.3389/fphys.2022.913063. eCollection 2022. Front Physiol. 2022. PMID: 35860655 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

