A Pilot Study to Assess the Feasibility of a Web-Based Survey to Examine Patient-Reported Symptoms and Satisfaction in Patients with Ankylosing Spondylitis Receiving Secukinumab
- PMID: 31054047
- PMCID: PMC6520413
- DOI: 10.1007/s40801-019-0154-4
A Pilot Study to Assess the Feasibility of a Web-Based Survey to Examine Patient-Reported Symptoms and Satisfaction in Patients with Ankylosing Spondylitis Receiving Secukinumab
Abstract
Purpose: This real-world study evaluated the feasibility of assessing patient-reported symptom improvement and treatment satisfaction using a web-based survey among patients with ankylosing spondylitis (AS) treated with secukinumab.
Methods: This cross-sectional, web-based survey collected data on demographics, symptoms, treatment history, and treatment satisfaction from US patients with AS who were receiving secukinumab at survey participation. Patients reported AS symptoms experienced before and after secukinumab initiation, time to symptom improvement, and satisfaction with secukinumab treatment.
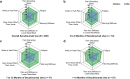
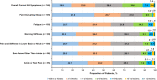
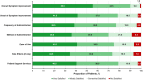
Results: Of 2755 patients screened, 200 with AS were included in the analysis. The mean (SD) age of patients was 34.4 (10.6) years; 86.5% were biologic experienced. Most (74.0%) reported overall improvement ("a little," "moderately," or "much better") in AS symptoms since secukinumab initiation compared with before secukinumab initiation; a similar trend was observed for all the individual symptoms analyzed (pain disrupting sleep, fatigue, morning stiffness, pain and stiffness in lower back or neck, sore areas other than joints, and ankle or heel pain [indicating enthesitis]). Approximately 41.9% of patients reported overall symptom improvement within 4 weeks of secukinumab treatment. Most expressed overall satisfaction ("very," "mostly," or "somewhat satisfied") with secukinumab regarding symptom improvement (99.0%), speed of symptom improvement (97.0%), frequency and method of administration (96.0% and 91.5%, respectively), ease of use (93.5%), patient support services (97.0%), and side effects, if any (93.0%).
Conclusion: Most patients reported overall symptom improvement and satisfaction with treatment. Our study indicates that patient-reported perspectives may be feasibly collected using a web-based survey to provide insights into treatment experience and satisfaction.
Conflict of interest statement
Conflicts of interest
M. Magrey served as a consultant for Novartis. M. Bozyczko has nothing to disclose. D. Wolin, M. Mordin, L. McLeod, E. Davenport, and C. Chirila are employees of RTI Health Solutions. Y. Park is an employee of Novartis.
Research involving human participants
An institutional review board exemption was obtained for the study prior to initiation of data collection.
Informed consent
Informed consent was obtained electronically from eligible patients interested in participating in this AS survey.
Figures
References
-
- Dean LE, Jones GT, MacDonald AG, et al. Global prevalence of ankylosing spondylitis. Rheumatology (Oxford) 2014;53:650–657. - PubMed
-
- Dougados M, Baeten D. Spondyloarthritis. Lancet. 2011;377:2127–2137. - PubMed
-
- Kang JH, Chen YH, Lin HC. Comorbidity profiles among patients with ankylosing spondylitis: a nationwide population-based study. Ann Rheum Dis. 2010;69:1165–1168. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

