A sexually dimorphic distribution of corticotropin-releasing factor receptor 1 in the paraventricular hypothalamus
- PMID: 31055007
- PMCID: PMC6897333
- DOI: 10.1016/j.neuroscience.2019.04.045
A sexually dimorphic distribution of corticotropin-releasing factor receptor 1 in the paraventricular hypothalamus
Erratum in
-
Corrigendum to "A Sexually Dimorphic Distribution of Corticotropin-Releasing Factor Receptor 1 in the Paraventricular Hypothalamus" [Neuroscience 409 (2019) 195-203].Neuroscience. 2020 Jan 21;428:1. doi: 10.1016/j.neuroscience.2019.12.013. Epub 2020 Jan 8. Neuroscience. 2020. PMID: 31923402 No abstract available.
Abstract
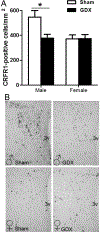
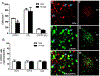
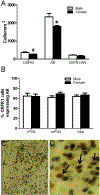
Sex differences in neural structures are generally believed to underlie sex differences reported in anxiety, depression, and the hypothalamic-pituitary-adrenal axis, although the specific circuitry involved is largely unclear. Using a corticotropin-releasing factor receptor 1 (CRFR1) reporter mouse line, we report a sexually dimorphic distribution of CRFR1 expressing cells within the paraventricular hypothalamus (PVN; males > females). Relative to adult levels, PVN CRFR1-expressing cells are sparse and not sexually dimorphic at postnatal days 0, 4, or 21. This suggests that PVN cells might recruit CRFR1 during puberty or early adulthood in a sex-specific manner. The adult sex difference in PVN CRFR1 persists in old mice (20-24 months). Adult gonadectomy (6 weeks) resulted in a significant decrease in CRFR1-immunoreactive cells in the male but not female PVN. CRFR1 cells show moderate co-expression with estrogen receptor alpha (ERα) and high co-expression with androgen receptor, indicating potential mechanisms through which circulating gonadal hormones might regulate CRFR1 expression and function. Finally, we demonstrate that a psychological stressor, restraint stress, induces a sexually dimorphic pattern of neural activation in PVN CRFR1 cells (males >females) as assessed by co-localization with the transcription/neural activation marker phosphorylated CREB. Given the known role of CRFR1 in regulating stress-associated behaviors and hormonal responses, this CRFR1 PVN sex difference might contribute to sex differences in these functions.
Keywords: androgen; corticotropin releasing factor; sex difference; stress.
Copyright © 2019 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures
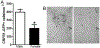



References
-
- Abel KB, Majzoub JA (2005), Molecular biology of the HPA axis. Ed. Steckler T, Kalin NH, and Reul JMHM. Techniques in Behavioral and Neural Sciences 15(1):79–94.
-
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ (2010), Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry 15(9):877, 896–904. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

