Respiratory frequency plasticity during development
- PMID: 31055188
- PMCID: PMC6561787
- DOI: 10.1016/j.resp.2019.04.014
Respiratory frequency plasticity during development
Abstract
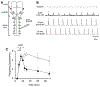
Respiratory frequency plasticity is a long-lasting increase in breathing frequency due to a perturbation. Mechanisms underlying respiratory frequency are poorly understood, and there is little evidence of frequency plasticity in neonates. This hybrid review/research article discusses available literature regarding frequency plasticity and highlights potential research opportunities. Also, we include data demonstrating a model of frequency plasticity using isolated neonatal rat brainstem-spinal cord preparations. Specifically, substance P (SubP) application induced a long-lasting (>60 min) increase in spontaneous respiratory motor burst frequency, particularly in brainstem-spinal cords with the pons attached; there were no male/female differences. SubP-induced frequency plasticity is dependent on the application pattern, such that intermittent (rather than sustained) SubP applications induce more frequency plasticity. SubP-induced frequency plasticity was blocked by a neurokinin-1 receptor antagonist. Thus, the newborn rat respiratory control system has the capacity to express frequency plasticity. Identifying mechanisms that induce frequency plasticity may lead to novel methods to safely treat breathing disorders in premature and newborn infants.
Keywords: Brain stem; Development; Frequency plasticity; Neonatal; Neuroplasticity; Respiratory; Spinal cord; Substance P.
Copyright © 2019 Elsevier B.V. All rights reserved.
Figures
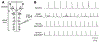


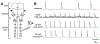


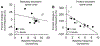
References
-
- Bach KB, Mitchell GS, 1998. Hypercapnia-induced long-term depression of respiratory activity requires alpha2-adrenergic receptors. J. Appl. Physiol 84, 2099–2105. - PubMed
-
- Bairam A, Laflamme N, Drolet C, Piedboeuf B, Shah PS, Kinkead R, 2018. Sex-based differences in apnoea of prematurity: a retrospective cohort study. Exp. Physiol 103, 1403–1411. - PubMed
-
- Bavis RW, MacFarlane PM, 2017. Developmental plasticity in the neural control of breathing. Exp. Neurol 287, 176–191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

