Epidermal autophagy and beclin 1 regulator 1 and loricrin: a paradigm shift in the prognostication and stratification of the American Joint Committee on Cancer stage I melanomas
- PMID: 31056744
- PMCID: PMC6973157
- DOI: 10.1111/bjd.18086
Epidermal autophagy and beclin 1 regulator 1 and loricrin: a paradigm shift in the prognostication and stratification of the American Joint Committee on Cancer stage I melanomas
Abstract
Background: The updated American Joint Committee on Cancer (AJCC) staging criteria for melanoma remain unable to identify high-risk stage I tumour subsets.
Objectives: To determine the utility of epidermal autophagy and beclin 1 regulator 1 (AMBRA1)/loricrin (AMLo) expression as a prognostic biomarker for AJCC stage I cutaneous melanoma.
Methods: Peritumoral AMBRA1 expression was evaluated in a retrospective discovery cohort of 76 AJCC stage I melanomas. AMLo expression was correlated with clinical outcomes up to 12 years in two independent powered, retrospective validation and qualification cohorts comprising 379 AJCC stage I melanomas.
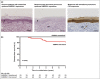
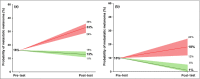
Results: Decreased AMBRA1 expression in the epidermis overlying primary melanomas in a discovery cohort of 76 AJCC stage I tumours was associated with a 7-year disease-free survival (DFS) rate of 81·5% vs. 100% survival with maintained AMBRA1 (P < 0·081). Following an immunohistochemistry protocol for semi-quantitative analysis of AMLo, analysis was undertaken in validation (n = 218) and qualification cohorts (n = 161) of AJCC stage I melanomas. Combined cohort analysis revealed a DFS rate of 98·3% in the AMLo low-risk group (n = 239) vs. 85·4% in the AMLo high-risk cohort (n = 140; P < 0·001). Subcohort multivariate analysis revealed that an AMLo hazard ratio (HR) of 4·04 [95% confidence interval (CI) 1·69-9·66; P = 0·002] is a stronger predictor of DFS than Breslow depth (HR 2·97, 95% CI 0·93-9·56; P = 0·068) in stage IB patients.
Conclusions: Loss of AMLo expression in the epidermis overlying primary AJCC stage I melanomas identifies high-risk tumour subsets independently of Breslow depth. What's already known about this topic? There is an unmet clinical need for biomarkers of early-stage melanoma. Autophagy and beclin 1 regulator 1 (AMBRA1) is a proautophagy regulatory protein with known roles in cell proliferation and differentiation, and is a known tumour suppressor. Loricrin is a marker of epidermal terminal differentiation. What does this study add? AMBRA1 has a functional role in keratinocyte/epidermal proliferation and differentiation. The combined decrease/loss of peritumoral AMBRA1 and loricrin is associated with a significantly increased risk of metastatic spread in American Joint Committee on Cancer (AJCC) stage I tumours vs. melanomas, in which peritumoral AMBRA1 and loricrin are maintained, independently of Breslow depth. What is the translational message? The integration of peritumoral epidermal AMBRA1/loricrin biomarker expression into melanoma care guidelines will facilitate more accurate, personalized risk stratification for patients with AJCC stage I melanomas, thereby facilitating stratification for appropriate follow-up and informing postdiagnostic investigations, including sentinel lymph node biopsy, ultimately resulting in improved disease outcomes and rationalization of healthcare costs.
© 2019 The Authors. British Journal of Dermatology published by John Wiley & Sons Ltd on behalf of British Association of Dermatologists.
Figures




Comment in
-
New biomarkers improve stratification of patients with melanoma.Br J Dermatol. 2020 Jan;182(1):5-6. doi: 10.1111/bjd.18555. Br J Dermatol. 2020. PMID: 31894877 No abstract available.
References
-
- National Cancer Institue . Surveillance, Epidemiology, and End Results Program. Available at: https://seer.cancer.gov/statfacts/html/melan.html (last accessed 29 April 2019).
-
- Nikolaou V, Stratigos AJ. Emerging trends in the epidemiology of melanoma. Br J Dermatol 2014; 170:11–19. - PubMed
-
- Edge SB, Byrd DR, Compton CC et al AJCC Cancer Staging Manual, 7th edn New York: Springer, 2010.
-
- Amin MB, Edge S, Greene F et al AJCC Cancer Staging Manual, 8th edn New York: Springer, 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

