Arterial Stiffness Due to Carotid Calcification Disrupts Cerebral Blood Flow Regulation and Leads to Cognitive Deficits
- PMID: 31057061
- PMCID: PMC6512142
- DOI: 10.1161/JAHA.118.011630
Arterial Stiffness Due to Carotid Calcification Disrupts Cerebral Blood Flow Regulation and Leads to Cognitive Deficits
Abstract
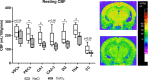
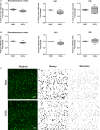
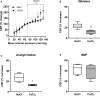
Background Arterial stiffness is associated with cognitive decline and dementia; however, the precise mechanisms by which it affects the brain remain unclear. Methods and Results Using a mouse model based on carotid calcification this study characterized mechanisms that could contribute to brain degeneration due to arterial stiffness. At 2 weeks postcalcification, carotid stiffness attenuated resting cerebral blood flow in several brain regions including the perirhinal/entorhinal cortex, hippocampus, and thalamus, determined by autoradiography ( P<0.05). Carotid calcification impaired cerebral autoregulation and diminished cerebral blood flow responses to neuronal activity and to acetylcholine, examined by laser Doppler flowmetry ( P<0.05, P<0.01). Carotid stiffness significantly affected spatial memory at 3 weeks ( P<0.05), but not at 2 weeks, suggesting that cerebrovascular impairments precede cognitive dysfunction. In line with the endothelial deficits, carotid stiffness led to increased blood-brain barrier permeability in the hippocampus ( P<0.01). This region also exhibited reductions in vessel number containing collagen IV ( P<0.01), as did the somatosensory cortex ( P<0.05). No evidence of cerebral microhemorrhages was present. Carotid stiffness did not affect the production of mouse amyloid-β (Aβ) or tau phosphorylation, although it led to a modest increase in the Aβ40/Aβ42 ratio in frontal cortex ( P<0.01). Conclusions These findings suggest that carotid stiffness alters brain microcirculation and increases blood-brain barrier permeability associated with cognitive impairments. Therefore, arterial stiffness should be considered a relevant target to protect the brain and prevent cognitive dysfunctions.
Keywords: arterial stiffness; blood‐brain barrier; carotid calcification; cerebral blood flow; cognitive impairment.
Figures



References
-
- Pase MP, Herbert A, Grima NA, Pipingas A, O'Rourke MF. Arterial stiffness as a cause of cognitive decline and dementia: a systematic review and meta‐analysis. Intern Med J. 2012;42:808–815. - PubMed
-
- Hanon O, Haulon S, Lenoir H, Seux ML, Rigaud AS, Safar M, Girerd X, Forette F. Relationship between arterial stiffness and cognitive function in elderly subjects with complaints of memory loss. Stroke. 2005;36:2193–2197. - PubMed
-
- Meyer ML, Palta P, Tanaka H, Deal JA, Wright J, Knopman DS, Griswold ME, Mosley TH, Heiss G. Association of central arterial stiffness and pressure pulsatility with mild cognitive impairment and dementia: the Atherosclerosis Risk in Communities Study‐Neurocognitive Study (ARIC‐NCS). J Alzheimers Dis. 2017;57:195–204. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

