Transmission of a Protease-Secreting Bacterial Symbiont Among Pea Aphids via Host Plants
- PMID: 31057424
- PMCID: PMC6479166
- DOI: 10.3389/fphys.2019.00438
Transmission of a Protease-Secreting Bacterial Symbiont Among Pea Aphids via Host Plants
Abstract
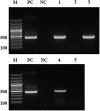
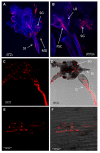
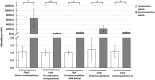
Aphids are economically important pest insects that damage plants by phloem feeding and the transmission of plant viruses. Their ability to feed exclusively on nutritionally poor phloem sap is dependent on the obligatory symbiotic bacterium Buchnera aphidicola, but additional facultative symbionts may also be present, a common example of which is Serratia symbiotica. Many Serratia species secrete extracellular enzymes, so we hypothesised that S. symbiotica may produce proteases that help aphids to feed on plants. Molecular analysis, including fluorescence in situ hybridization (FISH), revealed that S. symbiotica colonises the gut, salivary glands and mouthparts (including the stylet) of the pea aphid Acyrthosiphon pisum, providing a mechanism to transfer the symbiont into host plants. S. symbiotica was also detected in plant tissues wounded by the penetrating stylet and was transferred to naïve aphids feeding on plants containing this symbiont. The maintenance of S. symbiotica by repeated transmission via plants may explain the high frequency of this symbiont in aphid populations. Proteomic analysis of the supernatant from a related but cultivable S. symbiotica strain cultured in liquid medium revealed the presence of known and novel proteases including metalloproteases. The corresponding transcripts encoding these S. symbiotica enzymes were detected in A. pisum and in plants carrying the symbiont, although the mRNA was much more abundant in the aphids. Our data suggest that enzymes from S. symbiotica may facilitate the digestion of plant proteins, thereby helping to suppress plant defense, and that the symbionts are important mediators of aphid-plant interactions.
Keywords: Serratia symbiotica; Vicia faba; extracellular proteases; phloem sap; symbiosis.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

