Modeling Modulation of the Tick Regulome in Response to Anaplasma phagocytophilum for the Identification of New Control Targets
- PMID: 31057429
- PMCID: PMC6482211
- DOI: 10.3389/fphys.2019.00462
Modeling Modulation of the Tick Regulome in Response to Anaplasma phagocytophilum for the Identification of New Control Targets
Abstract
Ticks act as vectors of pathogens affecting human and animal health worldwide, and recent research has focused on the characterization of tick-pathogen interactions using omics technologies to identify new targets for developing novel control interventions. The regulome (transcription factors-target genes interactions) plays a critical role in cell response to pathogen infection. Therefore, the application of regulomics to tick-pathogen interactions would advance our understanding of these molecular interactions and contribute to the identification of novel control targets for the prevention and control of tick infestations and tick-borne diseases. However, limited information is available on the role of tick regulome in response to pathogen infection. In this study, we applied complementary in silico approaches to modeling how Anaplasma phagocytophilum infection modulates tick vector regulome. This proof-of-concept research provided support for the use of network analysis in the study of regulome response to infection, resulting in new information on tick-pathogen interactions and potential targets for developing interventions for the control of tick infestations and pathogen transmission. Deciphering the precise nature of circuits that shape the tick regulome in response to pathogen infection is an area of research that in the future will advance our knowledge of tick-pathogen interactions, and the identification of new antigens for the control of tick infestations and pathogen infection/transmission.
Keywords: Anaplasma phagocytophilum; ISE6 cells; Ixodes scapularis; regulome; tick; transcription; vaccine.
Figures
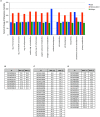
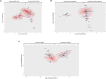

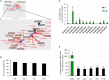
References
-
- Alberdi P., Ayllón N., Cabezas-Cruz A., Bell-Sakyi L., Zweygarth E., Stuen S., et al. (2015). Infection of Ixodes spp. tick cells with different Anaplasma phagocytophilum isolates induces the inhibition of apoptotic cell death. Ticks Tick Borne Dis. 6 758–767. 10.1016/j.ttbdis.2015.07.001 - DOI - PubMed
-
- Antunes S., Galindo R. C., Almazán C., Rudenko N., Golovchenko M., Grubhoffer L., et al. (2012). Functional genomics studies of Rhipicephalus (Boophilus) annulatus ticks in response to infection with the cattle protozoan parasite, Babesia bigemina. Int. J. Parasitol. 42 187–195. 10.1016/j.ijpara.2011.12.003 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources

