Assessing the Functional Relevance of Variants in the IKAROS Family Zinc Finger Protein 1 (IKZF1) in a Cohort of Patients With Primary Immunodeficiency
- PMID: 31057532
- PMCID: PMC6477086
- DOI: 10.3389/fimmu.2019.00568
Assessing the Functional Relevance of Variants in the IKAROS Family Zinc Finger Protein 1 (IKZF1) in a Cohort of Patients With Primary Immunodeficiency
Erratum in
-
Corrigendum: Assessing the Functional Relevance of Variants in the IKAROS Family Zinc Finger Protein 1 (IKZF1) in a Cohort of Patients With Primary Immunodeficiency.Front Immunol. 2019 Jun 28;10:1490. doi: 10.3389/fimmu.2019.01490. eCollection 2019. Front Immunol. 2019. PMID: 31316524 Free PMC article.
Abstract
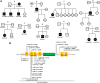
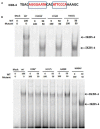
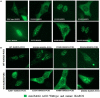
Common variable immunodeficiency (CVID) is the most frequent symptomatic primary immunodeficiency. Patients with CVID are prone to recurrent bacterial infection due to the failure of adequate immunoglobulin production. Monogenetic defects have been identified in ~25% of CVID patients. Recently, mutations in IKZF1, encoding the zinc-finger transcription factor IKAROS which is broadly expressed in hematopoietic cells, have been associated with a CVID-like phenotype. Herein we describe 11 patients with heterozygous IKZF1 variants from eight different families with autosomal dominant CVID and two siblings with an IKZF1 variant presenting with inflammatory bowel disease (IBD). This study shows that mutations affecting the DNA binding domain of IKAROS can impair the interaction with the target DNA sequence thereby preventing heterochromatin and pericentromeric localization (HC-PC) of the protein. Our results also indicate an impairment of pericentromeric localization of IKAROS by overexpression of a truncated variant, caused by an immature stop codon in IKZF1. We also describe an additional variant in TNFSF10, encoding Tumor Necrosis Factor Related Apoptosis Inducing Ligand (TRAIL), additionally presented in individuals of Family A. Our results indicate that this variant may impair the TRAIL-induced apoptosis in target cell lines and prohibit the NFκB activation by TRAIL and may act as a modifier in Family A.
Keywords: CVID; IKAROS; TRAIL DNA binding; monogenic defects; nuclear localization.
Figures

Similar articles
-
IKAROS-Associated Diseases in 2020: Genotypes, Phenotypes, and Outcomes in Primary Immune Deficiency/Inborn Errors of Immunity.J Clin Immunol. 2021 Jan;41(1):1-10. doi: 10.1007/s10875-020-00936-x. Epub 2021 Jan 3. J Clin Immunol. 2021. PMID: 33392855 Review.
-
A Point Mutation in IKAROS ZF1 Causes a B Cell Deficiency in Mice.J Immunol. 2021 Apr 1;206(7):1505-1514. doi: 10.4049/jimmunol.1901464. Epub 2021 Mar 3. J Immunol. 2021. PMID: 33658297 Free PMC article.
-
Abnormal SCID Newborn Screening and Spontaneous Recovery Associated with a Novel Haploinsufficiency IKZF1 Mutation.J Clin Immunol. 2021 Aug;41(6):1241-1249. doi: 10.1007/s10875-021-01035-1. Epub 2021 Apr 14. J Clin Immunol. 2021. PMID: 33855675 Free PMC article. Clinical Trial.
-
Loss of B Cells in Patients with Heterozygous Mutations in IKAROS.N Engl J Med. 2016 Mar 17;374(11):1032-1043. doi: 10.1056/NEJMoa1512234. N Engl J Med. 2016. PMID: 26981933 Free PMC article.
-
IKAROS Family Zinc Finger 1-Associated Diseases in Primary Immunodeficiency Patients.Immunol Allergy Clin North Am. 2020 Aug;40(3):461-470. doi: 10.1016/j.iac.2020.04.004. Immunol Allergy Clin North Am. 2020. PMID: 32654692 Free PMC article. Review.
Cited by
-
Update on Infections in Primary Antibody Deficiencies.Front Immunol. 2021 Feb 11;12:634181. doi: 10.3389/fimmu.2021.634181. eCollection 2021. Front Immunol. 2021. PMID: 33643318 Free PMC article. Review.
-
Germline IKAROS dimerization haploinsufficiency causes hematologic cytopenias and malignancies.Blood. 2021 Jan 21;137(3):349-363. doi: 10.1182/blood.2020007292. Blood. 2021. PMID: 32845957 Free PMC article.
-
Pathogenic germline IKZF1 variant alters hematopoietic gene expression profiles.Cold Spring Harb Mol Case Stud. 2021 Aug 2;7(4):a006015. doi: 10.1101/mcs.a006015. Print 2021 Aug. Cold Spring Harb Mol Case Stud. 2021. PMID: 34162668 Free PMC article.
-
IKAROS-Associated Diseases in 2020: Genotypes, Phenotypes, and Outcomes in Primary Immune Deficiency/Inborn Errors of Immunity.J Clin Immunol. 2021 Jan;41(1):1-10. doi: 10.1007/s10875-020-00936-x. Epub 2021 Jan 3. J Clin Immunol. 2021. PMID: 33392855 Review.
-
Multifaceted roles of IKZF1 gene, perspectives from bench to bedside.Front Oncol. 2024 Jun 24;14:1383419. doi: 10.3389/fonc.2024.1383419. eCollection 2024. Front Oncol. 2024. PMID: 38978740 Free PMC article. Review.
References
-
- Warnatz K, Denz A, Dräger R, Braun M, Groth C, Wolff-Vorbeck G, et al. . Severe deficiency of switched memory B cells (CD27+IgM−IgD−) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood. (2002) 99:1544–51. 10.1182/blood.V99.5.1544 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

