Myeloid Derived Suppressor Cells Interactions With Natural Killer Cells and Pro-angiogenic Activities: Roles in Tumor Progression
- PMID: 31057536
- PMCID: PMC6482162
- DOI: 10.3389/fimmu.2019.00771
Myeloid Derived Suppressor Cells Interactions With Natural Killer Cells and Pro-angiogenic Activities: Roles in Tumor Progression
Abstract
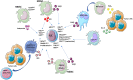
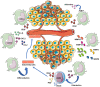
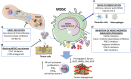
Myeloid-derived suppressor cells (MDSCs) contribute to the induction of an immune suppressive/anergic, tumor permissive environment. MDSCs act as immunosuppression orchestrators also by interacting with several components of both innate and adaptive immunity. Natural killer (NK) cells are innate lymphoid cells functioning as primary effector of immunity, against tumors and virus-infected cells. Apart from the previously described anergy and hypo-functionality of NK cells in different tumors, NK cells in cancer patients show pro-angiogenic phenotype and functions, similar to decidual NK cells. We termed the pro-angiogenic NK cells in the tumor microenvironment "tumor infiltrating NK" (TINKs), and peripheral blood NK cells in cancer patients "tumor associated NK" (TANKs). The contribution of MDSCs in regulating NK cell functions in tumor-bearing host, still represent a poorly explored topic, and even less is known on NK cell regulation of MDSCs. Here, we review whether the crosstalk between MDSCs and NK cells can impact on tumor onset, angiogenesis and progression, focusing on key cellular and molecular interactions. We also propose that the similarity of the properties of tumor associated/tumor infiltrating NK and MDSC with those of decidual NK and decidual MDSCs during pregnancy could hint to a possible onco-fetal origin of these pro-angiogenic leukocytes.
Keywords: angiogenesis; cytokines; decidua; myeloid derived suppressor cell (MDSC); natural killer cells (NK cells); tumor microenvironment.
Figures
Similar articles
-
Natural Killer Cell Interactions With Myeloid Derived Suppressor Cells in the Tumor Microenvironment and Implications for Cancer Immunotherapy.Front Immunol. 2021 May 5;12:633205. doi: 10.3389/fimmu.2021.633205. eCollection 2021. Front Immunol. 2021. PMID: 34025641 Free PMC article. Review.
-
Myeloid-derived suppressor cells depletion may cause pregnancy loss via upregulating the cytotoxicity of decidual natural killer cells.Am J Reprod Immunol. 2019 Apr;81(4):e13099. doi: 10.1111/aji.13099. Epub 2019 Mar 4. Am J Reprod Immunol. 2019. PMID: 30737988
-
Myeloid-Derived Suppressor Cells in Tumors: From Mechanisms to Antigen Specificity and Microenvironmental Regulation.Front Immunol. 2020 Jul 22;11:1371. doi: 10.3389/fimmu.2020.01371. eCollection 2020. Front Immunol. 2020. PMID: 32793192 Free PMC article. Review.
-
A think tank of TINK/TANKs: tumor-infiltrating/tumor-associated natural killer cells in tumor progression and angiogenesis.J Natl Cancer Inst. 2014 Sep 1;106(8):dju200. doi: 10.1093/jnci/dju200. Print 2014 Aug. J Natl Cancer Inst. 2014. PMID: 25178695 Free PMC article. Review.
-
Myeloid-Derived Suppressor Cells: Critical Cells Driving Immune Suppression in the Tumor Microenvironment.Adv Cancer Res. 2015;128:95-139. doi: 10.1016/bs.acr.2015.04.002. Epub 2015 May 12. Adv Cancer Res. 2015. PMID: 26216631 Free PMC article. Review.
Cited by
-
CAR-NK Cells in the Treatment of Solid Tumors.Int J Mol Sci. 2021 May 31;22(11):5899. doi: 10.3390/ijms22115899. Int J Mol Sci. 2021. PMID: 34072732 Free PMC article. Review.
-
Metamorphic Effect of Angiogenic Switch in Tumor Development: Conundrum of Tumor Angiogenesis Toward Progression and Metastatic Potential.Biomedicines. 2023 Jul 29;11(8):2142. doi: 10.3390/biomedicines11082142. Biomedicines. 2023. PMID: 37626639 Free PMC article. Review.
-
A Comprehensive Pan-Cancer Analysis of RBM8A Based on Data Mining.J Oncol. 2021 Jul 6;2021:9983354. doi: 10.1155/2021/9983354. eCollection 2021. J Oncol. 2021. PMID: 34326876 Free PMC article.
-
Bi-specific and Tri-specific NK Cell Engagers: The New Avenue of Targeted NK Cell Immunotherapy.Mol Diagn Ther. 2021 Sep;25(5):577-592. doi: 10.1007/s40291-021-00550-6. Epub 2021 Jul 29. Mol Diagn Ther. 2021. PMID: 34327614 Review.
-
Predicting Survival in Patients with Pancreatic Cancer by Integrating Bone Marrow FDG Uptake and Radiomic Features of Primary Tumor in PET/CT.Cancers (Basel). 2021 Jul 16;13(14):3563. doi: 10.3390/cancers13143563. Cancers (Basel). 2021. PMID: 34298775 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

