Cell Wall Invertase and Sugar Transporters Are Differentially Activated in Tomato Styles and Ovaries During Pollination and Fertilization
- PMID: 31057596
- PMCID: PMC6482350
- DOI: 10.3389/fpls.2019.00506
Cell Wall Invertase and Sugar Transporters Are Differentially Activated in Tomato Styles and Ovaries During Pollination and Fertilization
Abstract
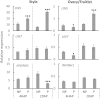
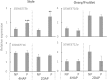
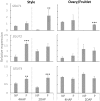
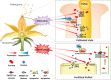
Flowering plants depend on pollination and fertilization to activate the transition from ovule to seed and ovary to fruit, namely seed and fruit set, which are key for completing the plant life cycle and realizing crop yield potential. These processes are highly energy consuming and rely on the efficient use of sucrose as the major nutrient and energy source. However, it remains elusive as how sucrose imported into and utilizated within the female reproductive organ is regulated in response to pollination and fertilization. Here, we explored this issue in tomato by focusing on genes encoding cell wall invertase (CWIN) and sugar transporters, which are major players in sucrose phloem unloading, and sink development. The transcript level of a major CWIN gene, LIN5, and CWIN activity were significantly increased in style at 4 h after pollination (HAP) in comparison with that in the non-pollination control, and this was sustained at 2 days after pollination (DAP). In the ovaries, however, CWIN activity and LIN5 expression did not increase until 2 DAP when fertilization occurred. Interestingly, a CWIN inhibitor gene INVINH1 was repressed in the pollinated style at 2 DAP. In response to pollination, the style exhibited increased expressions of genes encoding hexose transporters, SlHT1, 2, SlSWEET5b, and sucrose transporters SlSUT1, 2, and 4 from 4 HAP to 2 DAP. Upon fertilization, SlSUT1 and SlHT1 and 2, but not SlSWEETs, were also stimulated in fruitlets at 2 DAP. Together, the findings reveal that styles respond promptly and more broadly to pollination for activation of CWIN and sugar transporters to fuel pollen tube elongation, whereas the ovaries do not exhibit activation for some of these genes until fertilization occurs.
Highlights: Expression of genes encoding cell wall invertases and sugar transporters was stimulated in pollinated style and fertilized ovaries in tomato.
Keywords: cell wall invertase; fertilization; fruit set; pollen tube elongation; pollination; sugar transporter; sugar utilization; tomato.
Figures




References
-
- Büttner M., Truernit E., Baier K., Scholz-Starke J., Sontheim M., Lauterbach C., et al. (2000). AtSTP3, a green leaf-specific, low affinity monosaccharide-H+ symporter of Arabidopsis thaliana. Plant Cell Environ. 23 175–184. 10.1046/j.1365-3040.2000.00538.x - DOI
LinkOut - more resources
Full Text Sources

