Highly selective palladium-catalyzed one-pot, five-fold B-H/C-H cross coupling of monocarboranes with alkenes
- PMID: 31057746
- PMCID: PMC6471670
- DOI: 10.1039/c9sc00078j
Highly selective palladium-catalyzed one-pot, five-fold B-H/C-H cross coupling of monocarboranes with alkenes
Abstract
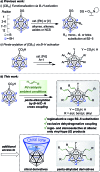
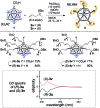
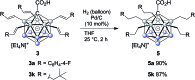
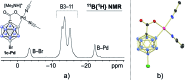
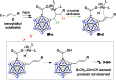
Palladium-catalyzed dehydrogenative B-H/C-H cross coupling of monocarborane anions with alkenes is reported, allowing for the first time the isolation of selectively penta-alkenylated boron clusters. The reaction cascade is regioselective for the cage positions, leading directly to B2-6 functionalization. Under mild and convenient conditions, styrenes, benzylic alkenes and aliphatic alkenes are demonstrated to be viable coupling partners with exclusive vinyl-type B-C bond formation. Multiple subsequent transformations provide access to directing group-free products, chiral derivatives and penta-alkylated cages. The five-fold coupling, combined with the latter reactions, represents a powerful methodology for the straightforward synthesis of new classes of boron clusters.
Figures
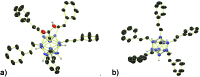
References
-
- Hosmane N. S., Boron Science: New Technologies and Applications, Taylor & Francis Books/CRC, Boca Raton, FL, 2011.
- Grimes R. N., Carboranes, Elsevier, Amsterdam, 3rd edn, 2016.
-
- Douvris C., Michl J. Chem. Rev. 2013;113:PR179–PR233. - PubMed
- Grimes R. N. Dalton Trans. 2015;44:5939–5956. - PubMed
- Núñez R., Tarrés M., Ferrer-Ugalde A., de Biani F. F., Teixidor F. Chem. Rev. 2016;116:14307–14378. - PubMed
- Núñez R., Romero I., Teixidor F., Viñas C. Chem. Soc. Rev. 2016;45:5147–5173. - PubMed
- Fischer S. P., Tomich A. W., Gui J., Lavallo V. Chem. Commun. 2019;55:1684–1701. - PubMed
-
-
For reviews and selected examples, see:
- Kim K.-C., Reed C. A., Elliott D. W., Mueller L. J., Tham F., Lin L., Lambert J. B. Science. 2002;297:825–827. - PubMed
- Krossing I., Raabe I. Angew. Chem., Int. Ed. 2004;43:2066–2090. - PubMed
- Kato T., Reed C. A. Angew. Chem., Int. Ed. 2004;43:2908–2911. - PubMed
- Douvris C., Ozerov O. V. Science. 2008;31:1188–1190. - PubMed
- Reed C. A. Acc. Chem. Res. 2010;43:121–128. - PMC - PubMed
- Allemann O., Duttwyler S., Romanato P., Baldridge K. K., Siegel J. S. Science. 2011;332:574–577. - PubMed
- Volkis V., Douvris C., Michl J. J. Am. Chem. Soc. 2011;133:7801–7809. - PubMed
- Knapp C., Weakly Coordinating Anions: Halogenated Borates and Dodecaborates, in Comprehensive Inorganic Chemistry II, Elsevier, Amsterdam, 2013, vol. 1, pp. 651–679.
- Kordts N., Borner C., Panisch R., Saak W., Müller T. Organometallics. 2014;33:1492–1498.
- Shoji Y., Tanaka N., Mikami K., Uchiyama M., Fukushima T. Nat. Chem. 2014;6:498–503. - PubMed
- Osman K. M., Powell D. R., Wehmschulte R. J. Inorg. Chem. 2015;54:9195–9200. - PubMed
- Kitazawa Y., Takita R., Yoshida K., Muranaka A., Matsubara S., Uchiyama M. J. Org. Chem. 2017;82:1931–1935. - PubMed
- Pell C. J., Zhu Y., Huacuja R., Herbert D. E., Hughes R. P., Ozerov O. V. Chem. Sci. 2017;8:3178–3186. - PMC - PubMed
- Shao B., Bagdasarian A. L., Popov S., Nelson H. M. Science. 2017;355:403–1407. - PubMed
- Müller T., Natalie K., Sandra K., Rathjen S., Sieling T., Großekappenberg H., Schmidtmann M. Chem.–Eur. J. 2017;23:10068–10079. - PubMed
- Omann L., Pudasaini B., Irran E., Klare H. F. T., Baik M.-H., Oestreich M. Chem. Sci. 2018;9:5600–5607. - PMC - PubMed
- Wu Q., Qu Z.-W., Omann L., Irran E., Klare H. F. T., Oestreich M. Angew. Chem., Int. Ed. 2018;57:9176–9179. - PubMed
- Riddlestone I. M., Kraft A., Schaefer J., Krossing I. Angew. Chem., Int. Ed. 2018;57:13982–14024. - PubMed
-
-
-
For selected publications, see:
- Finze M., Sprenger J. A. P., Schaack B. B. Dalton Trans. 2010;39:2708–2716. - PubMed
- Spokoyny A. M., Machan C. W., Clingerman D. J., Rosen M. S., Wiester M. J., Kennedy R. D., Stern C. L., Sarjeant A. A., Mirkin C. A. Nat. Chem. 2011;3:590–596. - PubMed
- El-Zaria M. E., Arii H., Nakamura H. Inorg. Chem. 2011;50:4149–4161. - PubMed
- Yao Z.-J., Jin G.-X. Coord. Chem. Rev. 2013;257:2522–2535.
- El-Hellani A., Lavallo V. Angew. Chem., Int. Ed. 2014;53:4489–4493. - PubMed
- Riley L. E., Chan A. P. Y., Taylor J., Man W. Y., Ellis D., Rosair G. M., Welch A. J., Sivaev I. B. Dalton Trans. 2016;45:1127–1137. - PubMed
- Holmes J., Pask C. M., Fox M. A., Willans C. E. Chem. Commun. 2016;52:6443–6446. - PubMed
- Estrada J., Lugo C. A., McArthur S. G., Lavallo V. Chem. Commun. 2016;52:1824–1826. - PubMed
- Šembera F., Plutnar J., Higelin A., Janoušek Z., Císařová I., Michl J. Inorg. Chem. 2016;55:3797–3806. - PubMed
- Zhou Y.-P., Raoufmoghaddam S., Szilvási T., Driess M. Angew. Chem., Int. Ed. 2016;55:12868–12872. - PubMed
- Coburger P., Schulz J., Klose J., Schwarze B., Sárosi M. B., Hey-Hawkins E. Inorg. Chem. 2017;56:292–304. - PubMed
- Selg C., Neumann W., Lönnecke P., Hey-Hawkins E., Zeitler K. Chem.–Eur. J. 2017;23:7932–7937. - PubMed
- Ilie A., Crespo O., Gimeno M. C., Holthausen M. C., Laguna A., Diefenbach M., Silvestru C. Eur. J. Inorg. Chem. 2017:2643–2652.
- Estrada J., Lavallo V. Angew. Chem., Int. Ed. 2017;56:9906–9909. - PubMed
- Cui P.-F., Lin Y.-J., Jin G.-X. Dalton Trans. 2017;46:15535–15540. - PubMed
- Eleazer B. J., Smith M. D., Popov A. A., Peryshkov D. V. Chem. Sci. 2018;9:2601–2608. - PMC - PubMed
- Rädisch T., Harmgarth N., Liebing P., Beltrán-Leiva M. J., Páez-Hernández D., Arratia-Pérez R., Engelhardt F., Hilfert L., Oehler F., Bussea S., Edelmann F. T. Dalton Trans. 2018;47:6666–6671. - PubMed
- Zhang Y., Zhang Z.-X., Wang Y., Li H., Bai F.-Q., Zhang H.-X. Inorg. Chem. Front. 2018;5:1016–1025.
- Oleshkevich E., Romero I., Teixidor F., Viñas C. Dalton Trans. 2018;47:14785–14798. - PubMed
-
-
- Han Y.-F., Jin G.-X. Acc. Chem. Res. 2014;47:3571–3579. - PubMed
- Housecroft C. E. J. Organomet. Chem. 2015;798:218–228.
- Estrada J., Lee S. E., McArthur S. G., El-Hellani A., Tham F. S., Lavallo V. J. Organomet. Chem. 2015;798:214–217.
- Clingerman D. J., Morris W., Mondloch J. E., Kennedy R. D., Sarjeant A. A., Stern C., Hupp J. T., Farhaab O. K., Mirkin C. A. Chem. Commun. 2015;51:6521–6523. - PubMed
- Ďordovič V., Tošner Z., Uchman M., Zhigunov A., Reza M., Ruokolainen J., Pramanik G., Cígler P., Kalíková K., Gradzielski M., Matějíček P. Langmuir. 2016;32:6713–6722. - PubMed
- Rodríguez-Hermida S., Tsang M. Y., Vignatti C., Stylianou K. C., Guillerm V., Pérez-Carvajal J., Teixidor F., Viñas C., Choquesillo-Lazarte D., Verdugo-Escamilla C., Peral I., Juanhuix J., Verdaguer A., Imaz I., Maspoch D., Planas J. G. Angew. Chem., Int. Ed. 2016;55:16049–16053. - PubMed
- Oleshkevich E., Viñas C., Romero I., Choquesillo-Lazarte D., Haukka M., Teixidor F. Inorg. Chem. 2017;56:5502–5505. - PubMed
- Xiong H., Zhou D., Zheng X., Zheng Y., Qi Y., Wang Y., Jing X., Huang Y. Chem. Commun. 2017;53:3422–3425. - PubMed
- Zhang K., Shen Y., Liu J., Spingler B., Duttwyler S. Chem. Commun. 2018;54:1698–1701. - PubMed
LinkOut - more resources
Full Text Sources

