Anisotropic strain release in a thermosalient crystal: correlation between the microscopic orientation of molecular rearrangements and the macroscopic mechanical motion
- PMID: 31057747
- PMCID: PMC6471989
- DOI: 10.1039/c8sc05563g
Anisotropic strain release in a thermosalient crystal: correlation between the microscopic orientation of molecular rearrangements and the macroscopic mechanical motion
Abstract
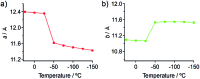
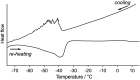
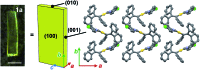
The salient effect, which refers to a jumping phenomenon of organic and organometallic molecular crystals typically triggered by phase transitions in response to external stimuli, has been investigated intensively in the last five years. A challenging topic in this research area is the question of how to characterize the release of microscopic strain accumulated during phase transitions, which generates macroscopic mechanical motion. Herein, we describe the thermosalient effect of the triphenylethenyl gold 4-chlorophenyl isocyanide complex 1, which jumps reversibly at approximately -100 °C upon cooling at 50 °C min-1 and heating at 30 °C min-1. Single-crystal X-ray diffraction measurements and differential scanning calorimetric analyses of 1 suggest the occurrence of a thermal phase transition at this temperature. Detailed structural analyses indicate that anisotropic changes to the molecular arrangement occur in 1, whereby the crystallographic a axis contracts upon cooling while the b axis expands. Simultaneously, macroscopic changes of the crystal dimensions occur. This is observed as bending, i.e., as an inclination of the crystal edges, and in the form of splitting, which occurs in a perpendicular direction to the major crystal axis. This study thus bridges the gap between macroscopic mechanical responses that are observed in high-speed photographic images and microscopic changes of the crystal structure, which are evaluated by X-ray diffraction measurements with face indexing.
Figures







References
-
- Sahoo S. C., Sinha S. B., Kiran M. S., Ramamurty U., Dericioglu A. F., Reddy C. M., Naumov P. J. Am. Chem. Soc. 2013;135:13843–13850. - PubMed
- Sahoo S. C., Panda M. K., Nath N. K., Naumov P. J. Am. Chem. Soc. 2013;135:12241–12251. - PubMed
- Chandra Sahoo S., Nath N. K., Zhang L., Semreen M. H., Al-Tel T. H., Naumov P. RSC Adv. 2014;4:7640–7647.
- Panda M. K., Runcevski T., Chandra Sahoo S., Belik A. A., Nath N. K., Dinnebier R. E., Naumov P. Nat. Commun. 2014;5:4811. - PubMed
- Panda M. K., Runcevski T., Husain A., Dinnebier R. E., Naumov P. J. Am. Chem. Soc. 2015;137:1895–1902. - PubMed
- Nauha E., Naumov P., Lusi M. CrystEngComm. 2016;18:4699–4703.
- Panda M. K., Centore R., Causa M., Tuzi A., Borbone F., Naumov P. Sci. Rep. 2016;6:29610. - PMC - PubMed
- Takeda T., Akutagawa T. Chem.–Eur. J. 2016;22:7763–7770. - PubMed
- Karothu D. P., Weston J., Desta I. T., Naumov P. J. Am. Chem. Soc. 2016;138:13298–13306. - PubMed
- Khalil A., Ahmed E., Naumov P. Chem. Commun. 2017;53:8470–8473. - PubMed
- Mittapalli S., Sravanakumar Perumalla D., Nangia A. IUCrJ. 2017;4:243–250. - PMC - PubMed
- Lončarić I., Popović J., Despoja V., Burazer S., Grgičević I., Popović D., Skoko ž. Cryst. Growth Des. 2017;17:4445–4453.
- Shibuya Y., Itoh Y., Aida T. Chem.–Asian J. 2017;12:811–815. - PubMed
- Rawat H., Samanta R., Bhattacharya B., Deolka S., Dutta A., Dey S., Raju K. B., Reddy C. M. Cryst. Growth Des. 2018;18:2918–2923.
- Alimi L. O., van Heerden D. P., Lama P., Smith V. J., Barbour L. J. Chem. Commun. 2018;54:6208–6211. - PubMed
-
- Naumov P., Sahoo S. C., Zakharov B. A., Boldyreva E. V. Angew. Chem., Int. Ed. 2013;52:9990–9995. - PubMed
- Medishetty R., Husain A., Bai Z., Runcevski T., Dinnebier R. E., Naumov P., Vittal J. J. Angew. Chem., Int. Ed. 2014;53:5907–5911. - PubMed
- Medishetty R., Sahoo S. C., Mulijanto C. E., Naumov P., Vittal J. J. Chem. Mater. 2015;27:1821–1829.
- Commins P., Natarajan A., Tsai C.-K., Khan S. I., Nath N. K., Naumov P., Garcia-Garibay M. A. Cryst. Growth Des. 2015;15:1983–1990.
- Seki T., Sakurada K., Muromoto M., Ito H. Chem. Sci. 2015;6:1491–1497. - PMC - PubMed
- Mishra M. K., Mukherjee A., Ramamurty U., Desiraju G. R. IUCrJ. 2015;2:653–660. - PMC - PubMed
- Hatano E., Morimoto M., Hyodo K., Yasuda N., Yokojima S., Nakamura S., Uchida K. Chem.–Eur. J. 2016;22:12680–12683. - PubMed
- Mulijanto C. E., Quah H. S., Tan G. K., Donnadieu B., Vittal J. J. IUCrJ. 2017;4:65–71. - PMC - PubMed
- Hatano E., Morimoto M., Imai T., Hyodo K., Fujimoto A., Nishimura R., Sekine A., Yasuda N., Yokojima S., Nakamura S., Uchida K. Angew. Chem., Int. Ed. 2017;56:12576–12580. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

