A versatile catalyst system for enantioselective synthesis of 2-substituted 1,4-benzodioxanes
- PMID: 31057761
- PMCID: PMC6472100
- DOI: 10.1039/c8sc05612a
A versatile catalyst system for enantioselective synthesis of 2-substituted 1,4-benzodioxanes
Abstract
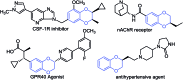
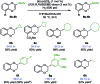
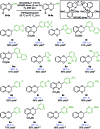
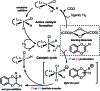
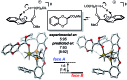
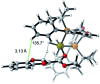
We report the synthesis of enantiomerically enriched 1,4-benzodioxanes containing alkyl, aryl, heteroaryl, and/or carbonyl substituents at the 2-position. The starting 1,4-benzodioxines were readily synthesized via ring closing metathesis using an efficient nitro-Grela catalyst at ppm levels. Excellent enantioselectivities of up to 99:1 er were obtained by using the versatile catalyst system [Ir(cod)Cl]2/BIDIME-dimer in the asymmetric hydrogenation of 2-substituted 1,4-benzodioxines. Furthermore, DFT calculations reveal that the selectivity of the process is controlled by the protonation step; and coordinating groups on the substrate may alter the interaction with the catalyst, resulting in a change in the facial selectivity.
Figures





Similar articles
-
Highly Efficient Asymmetric Hydrogenation Catalyzed by Iridium Complexes with Tridentate Chiral Spiro Aminophosphine Ligands.Acc Chem Res. 2023 Feb 7;56(3):332-349. doi: 10.1021/acs.accounts.2c00764. Epub 2023 Jan 23. Acc Chem Res. 2023. PMID: 36689780
-
Synthesis of trans-(3S)-amino-(4R)-alkyl- and -(4S)-aryl-piperidines via ring-closing metathesis reaction.Org Lett. 2002 Dec 12;4(25):4499-502. doi: 10.1021/ol027019m. Org Lett. 2002. PMID: 12465922
-
Enantioselective Carbonyl 1,2- or 1,4-Addition Reactions of Nucleophilic Silyl and Diazo Compounds Catalyzed by the Chiral Oxazaborolidinium Ion.Acc Chem Res. 2019 Aug 20;52(8):2349-2360. doi: 10.1021/acs.accounts.9b00279. Epub 2019 Jul 17. Acc Chem Res. 2019. PMID: 31314494
-
Benzothiazoline: versatile hydrogen donor for organocatalytic transfer hydrogenation.Acc Chem Res. 2015 Feb 17;48(2):388-98. doi: 10.1021/ar500414x. Epub 2015 Jan 22. Acc Chem Res. 2015. PMID: 25611073 Review.
-
Stereoelectronic Effects in Ligand Design: Enantioselective Rhodium-Catalyzed Hydrogenation of Aliphatic Cyclic Tetrasubstituted Enamides and Concise Synthesis of (R)-Tofacitinib.Angew Chem Int Ed Engl. 2019 Sep 16;58(38):13573-13583. doi: 10.1002/anie.201908089. Epub 2019 Aug 13. Angew Chem Int Ed Engl. 2019. PMID: 31343811 Review.
Cited by
-
Cu-Catalyzed Asymmetric Aminoboration of E-Vinylarenes with pivZPhos as the Ligand.Org Lett. 2019 Nov 15;21(22):8952-8956. doi: 10.1021/acs.orglett.9b03328. Epub 2019 Oct 24. Org Lett. 2019. PMID: 31647668 Free PMC article.
-
Nitro and Other Electron Withdrawing Group Activated Ruthenium Catalysts for Olefin Metathesis Reactions.Angew Chem Int Ed Engl. 2021 Jun 14;60(25):13738-13756. doi: 10.1002/anie.202008150. Epub 2020 Dec 3. Angew Chem Int Ed Engl. 2021. PMID: 32808704 Free PMC article. Review.
-
Rational Design of New Dihydrobenzooxophosphole-Based Lewis Base Organocatalysts.Synlett. 2020 Apr;31(6):587-591. doi: 10.1055/s-0039-1690851. Synlett. 2020. PMID: 33542591 Free PMC article.
References
-
- Pilkington L. I., Barker D. Nat. Prod. Rep. 2015;32:1369. - PubMed
- Abeywardane A.; Burke M. J.; Kirrane T. M.; Netherton M. R.; Padyana A. K.; Smith Keenan L. L.; Takahashi H.; Turner M. R.; Zhang Q.; Zhang Q., Benzodioxane Inhibitors of Leukotrien Production, International Patent WO 2012125598 A1, September 9, 2012.
- Zhou R., Luo G., Ewing A. G. J. Neurosci. 1994;14:2402. - PMC - PubMed
- Arnoldi A., Bassoli A., Merlini L., Ragg E. J. Chem. Soc., Perkin Trans. 1. 1993:1359.
- Hikino H., Kiso Y., Wagner H., Fiebig M. Planta Med. 1984;50:248. - PubMed
- Zhuang L.-G., Seligmann O., Jurcic K., Wagner H. Planta Med. 1982;45:172. - PubMed
-
- Patel S. D., Habeski W. M., Min H., Zhang J. S., Roof R., Snyder B., Bora G., Campbell B., Li C., Hidayetoglu D., Johnson D. S., Chaudhry A., Charlton M. E., Kablaoui N. M. Bioorg. Med. Chem. Lett. 2008;18:5689. - PubMed
-
- Tomiyama T., Wakabayashi S., Yokota M. J. Med. Chem. 1989;32:1988. - PubMed
-
- Ertan R., Göker H. FABAD J. Pharm. Sci. 1987;12:152.
-
- Hibert M. F., Gittos M. W., Middlemiss D. N., Mir A. K., Fozard J. R. J. Med. Chem. 1988;31:1087. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

