A versatile catalyst system for enantioselective synthesis of 2-substituted 1,4-benzodioxanes
- PMID: 31057761
- PMCID: PMC6472100
- DOI: 10.1039/c8sc05612a
A versatile catalyst system for enantioselective synthesis of 2-substituted 1,4-benzodioxanes
Abstract
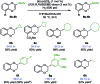
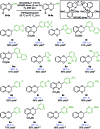
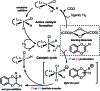
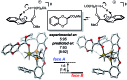
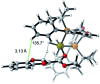
We report the synthesis of enantiomerically enriched 1,4-benzodioxanes containing alkyl, aryl, heteroaryl, and/or carbonyl substituents at the 2-position. The starting 1,4-benzodioxines were readily synthesized via ring closing metathesis using an efficient nitro-Grela catalyst at ppm levels. Excellent enantioselectivities of up to 99:1 er were obtained by using the versatile catalyst system [Ir(cod)Cl]2/BIDIME-dimer in the asymmetric hydrogenation of 2-substituted 1,4-benzodioxines. Furthermore, DFT calculations reveal that the selectivity of the process is controlled by the protonation step; and coordinating groups on the substrate may alter the interaction with the catalyst, resulting in a change in the facial selectivity.
Figures





References
-
- Pilkington L. I., Barker D. Nat. Prod. Rep. 2015;32:1369. - PubMed
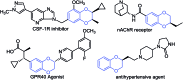
- Abeywardane A.; Burke M. J.; Kirrane T. M.; Netherton M. R.; Padyana A. K.; Smith Keenan L. L.; Takahashi H.; Turner M. R.; Zhang Q.; Zhang Q., Benzodioxane Inhibitors of Leukotrien Production, International Patent WO 2012125598 A1, September 9, 2012.
- Zhou R., Luo G., Ewing A. G. J. Neurosci. 1994;14:2402. - PMC - PubMed
- Arnoldi A., Bassoli A., Merlini L., Ragg E. J. Chem. Soc., Perkin Trans. 1. 1993:1359.
- Hikino H., Kiso Y., Wagner H., Fiebig M. Planta Med. 1984;50:248. - PubMed
- Zhuang L.-G., Seligmann O., Jurcic K., Wagner H. Planta Med. 1982;45:172. - PubMed
-
- Patel S. D., Habeski W. M., Min H., Zhang J. S., Roof R., Snyder B., Bora G., Campbell B., Li C., Hidayetoglu D., Johnson D. S., Chaudhry A., Charlton M. E., Kablaoui N. M. Bioorg. Med. Chem. Lett. 2008;18:5689. - PubMed
-
- Tomiyama T., Wakabayashi S., Yokota M. J. Med. Chem. 1989;32:1988. - PubMed
-
- Ertan R., Göker H. FABAD J. Pharm. Sci. 1987;12:152.
-
- Hibert M. F., Gittos M. W., Middlemiss D. N., Mir A. K., Fozard J. R. J. Med. Chem. 1988;31:1087. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

