Peptide-based capsules with chirality-controlled functionalized interiors - rational design and amplification from dynamic combinatorial libraries
- PMID: 31057768
- PMCID: PMC6482442
- DOI: 10.1039/c8sc05455j
Peptide-based capsules with chirality-controlled functionalized interiors - rational design and amplification from dynamic combinatorial libraries
Abstract
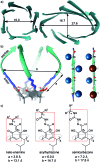
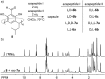
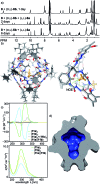
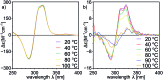
Peptides are commonly perceived as inapplicable components for construction of porous structures. Due to their flexibility the design is difficult and shape persistence of such putative structures is diminished. Notwithstanding these limitations, the advantages of peptides as building blocks are numerous: they are functional and functionalizable, widely available, diverse and biocompatible. We aimed at the construction of discrete porous structures that exploit the inherent functionality of peptides by an approach that is inspired by nature: structural pockets are defined by the backbones of peptides while functionality is introduced by their side chains. In this work peptide ribbons were preorganized on a macrocyclic scaffold using azapeptide-aldehyde reactions. The resulting cavitands with semicarbazone linkers arrange the peptide backbones at positions that are suitable for self-assembly of dimeric capsules by formation of binding motifs that resemble eight-stranded β-barrels. Self-assembly properties and inside/outside positions of the side chains depend crucially on the chirality of peptides. By rational optimization of successive generations of capsules we have found that azapeptides containing three amino acids in a (l, d, d) sequence give well-defined dimeric capsules with side chains inside their cavities. Taking advantage of the reversibility of the reaction of semicarbazone formation we have also employed the dynamic covalent chemistry (DCC) for a combinatorial discovery of capsules that could not be rationally designed. Indeed, the results show that stable capsules with side chains positioned internally can be obtained even for shorter sequences but only for combination peptides of (l, l) and (d, l) chirality. The hybrid (l, l)(d, l) capsule is amplified directly from a reaction mixture containing two different peptides. All capsules gain substantial ordering upon self-assembly, which is manifested by a two orders of magnitude increase of the intensity of CD spectra of capsules compared with non-assembled analogs. Temperature-dependent CD measurements indicate that the capsules remain stable over the entire temperature range tested (20-100 °C). Circular dichroism coupled with TD DFT calculations, DOSY measurements and X-ray crystallography allow for elucidation of the structures in the solid state and in solution and guide their iterative evolution for the current goals.
Figures






Similar articles
-
Dynamic formation of hybrid peptidic capsules by chiral self-sorting and self-assembly.Angew Chem Int Ed Engl. 2014 Dec 8;53(50):13760-4. doi: 10.1002/anie.201407802. Epub 2014 Oct 8. Angew Chem Int Ed Engl. 2014. PMID: 25298130
-
Azapeptide Synthesis Methods for Expanding Side-Chain Diversity for Biomedical Applications.Acc Chem Res. 2017 Jul 18;50(7):1541-1556. doi: 10.1021/acs.accounts.7b00114. Epub 2017 Jun 9. Acc Chem Res. 2017. PMID: 28598597
-
Amplification of Electronic Circular Dichroism-A Tool to Follow Self-Assembly of Chiral Molecular Capsules.Molecules. 2021 Nov 24;26(23):7100. doi: 10.3390/molecules26237100. Molecules. 2021. PMID: 34885682 Free PMC article.
-
The world of beta- and gamma-peptides comprised of homologated proteinogenic amino acids and other components.Chem Biodivers. 2004 Aug;1(8):1111-239. doi: 10.1002/cbdv.200490087. Chem Biodivers. 2004. PMID: 17191902 Review.
-
Gabapentin: a stereochemically constrained gamma amino acid residue in hybrid peptide design.Acc Chem Res. 2009 Oct 20;42(10):1628-39. doi: 10.1021/ar9001153. Acc Chem Res. 2009. PMID: 19572698 Review.
Cited by
-
Rapid Access to Chiral and Tripodal Cavitands from β-Pinene.Chemistry. 2022 Dec 27;28(72):e202202416. doi: 10.1002/chem.202202416. Epub 2022 Nov 7. Chemistry. 2022. PMID: 36168151 Free PMC article.
-
Rational design of hyperstable antibacterial peptides for food preservation.NPJ Sci Food. 2021 Sep 1;5(1):26. doi: 10.1038/s41538-021-00109-z. NPJ Sci Food. 2021. PMID: 34471114 Free PMC article.
-
Expanding structural diversity in a library of disulfide macrocycles through in-situ imide hydrolysis.Sci Rep. 2022 Jan 7;12(1):38. doi: 10.1038/s41598-021-03944-y. Sci Rep. 2022. PMID: 34997018 Free PMC article.
-
Specific Noncovalent Association of Truncated exo-Functionalized Triangular Homochiral Isotrianglimines through Head-to-Head, Tail-to-Tail, and Honeycomb Supramolecular Motifs.J Org Chem. 2022 Mar 4;87(5):2356-2366. doi: 10.1021/acs.joc.1c02238. Epub 2022 Jan 14. J Org Chem. 2022. PMID: 35029991 Free PMC article.
-
Embracing Complexity: Peptides as Tunable Scaffolds in the Construction of Discrete Supramolecular Systems.Angew Chem Int Ed Engl. 2025 Aug 18;64(34):e202512014. doi: 10.1002/anie.202512014. Epub 2025 Jul 25. Angew Chem Int Ed Engl. 2025. PMID: 40631436 Free PMC article. Review.
References
-
- Furukawa H., Cordova K. E., O'Keeffe M., Yaghi O. M. Science. 2013;341:974. - PubMed
-
- Ding S.-Y., Wang W. Chem. Soc. Rev. 2013;42:548. - PubMed
-
- Tian J., Wang H., Zhang D.-W., Liu Y., Li Z.-T. Natl. Sci. Rev. 2017;4:426.
-
- Liu J., Chen L., Cui H., Zhang J., Zhang L., Su C.-Y., Chem. Soc. Rev., 2014, 43 , 6011 , , references 62–81 . - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

