Secondary amines as coupling partners in direct catalytic asymmetric reductive amination
- PMID: 31057780
- PMCID: PMC6482873
- DOI: 10.1039/c9sc00323a
Secondary amines as coupling partners in direct catalytic asymmetric reductive amination
Abstract
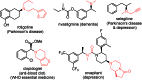
The secondary amine participating asymmetric reductive amination remains an unsolved problem in organic synthesis. Here we show for the first time that secondary amines are capable of effectively serving as N-sources in direct asymmetric reductive amination to afford corresponding tertiary chiral amines with the help of a selected additive set under mild conditions (0-25 °C). The applied chiral phosphoramidite ligands are readily prepared from BINOL and easily modified. Compared with common tertiary chiral amine synthetic methods, this procedure is much more concise and scalable, as exemplified by the facile synthesis of rivastigmine and N-methyl-1-phenylethanamine.
Figures
References
-
- Jarvis L. M. Chem. Eng. News. 2016;94:12–17.
-
- Roughley S. D., Jordan A. M. J. Med. Chem. 2011;54:3451–3479. - PubMed
-
- Chiral Amine Synthesis: Methods, Developments and Applications, ed. T. C. Nugent, Wiley-VCH, Weinheim, 2010.
- Stereoselective Formation of Amines, Top. Curr. Chem., ed. W. Li and X. Zhang, 2014, p. 343. - PubMed
- Blakemore D. C., Castro L., Churcher I., Rees D. C., Thomas A. W., Wilson D. M., Wood A. Nat. Chem. 2018;10:383–394. - PubMed
-
- Nugent T. C., El-Shazly M. Adv. Synth. Catal. 2010;352:753–819.
- Xie J.-H., Zhu S.-F., Zhou Q.-L. Chem. Rev. 2011;111:1713–1760. - PubMed
- Xie J.-H., Zhu S.-F., Zhou Q.-L. Chem. Soc. Rev. 2012;41:4126–4139. - PubMed
- Ager D. J., de Vries A. H. M., de Vries J. G. Chem. Soc. Rev. 2012;41:3340–3380. - PubMed
-
- Magee M. P., Norton J. R. J. Am. Chem. Soc. 2001;123:1778–1779. - PubMed
- Ji Y., Feng G.-S., Chen M.-W., Shi L., Du H., Zhou Y.-G. Org. Chem. Front. 2017;4:1125–1129.
LinkOut - more resources
Full Text Sources
Other Literature Sources