Morphological Characteristics of the Thymus and Spleen and the Subpopulation Composition of Lymphocytes in Peripheral Blood during Systemic Inflammatory Response in Male Rats with Different Resistance to Hypoxia
- PMID: 31057785
- PMCID: PMC6463648
- DOI: 10.1155/2019/7584685
Morphological Characteristics of the Thymus and Spleen and the Subpopulation Composition of Lymphocytes in Peripheral Blood during Systemic Inflammatory Response in Male Rats with Different Resistance to Hypoxia
Abstract
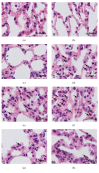
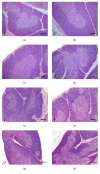
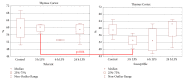
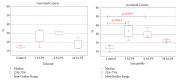
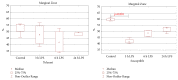
On the model of the systemic inflammatory response (SIRS), induced by lipopolysaccharide (LPS), the morphological and functional changes in the thymus and spleen and the subpopulation composition of peripheral blood lymphocytes of rats differing in resistance to hypoxia were studied. It was demonstrated that the level of endotoxin in blood serum after 3 hours of LPS administration in susceptible-to-hypoxia rats was 64 times higher than in the control group, while in tolerant-to-hypoxia animals it was only 8 times higher in 6 hours. After 24 hours of LPS injection, only in susceptible-to-hypoxia rats did the level of C-reactive protein in blood serum increase. There is a difference in the dynamics of morphological changes of lymphoid organs after LPS injection in tolerant- and susceptible-to-hypoxia animals. After 3 hours of LPS administration, the tolerant-to-hypoxia rats showed no changes in the thymus, spleen, and subpopulation composition of lymphocytes in peripheral blood. After 6 hours there was only a decrease in B-lymphocytes and increase in cytotoxic T-lymphocytes and NK cells. After 1 day of LPS injection, the tolerant-to-hypoxia rats had devastation in PALS of the spleen. After 3 hours of LPS injection the susceptible-to-hypoxia animals had reactive changes in the lymphoid organs: decrease of the thymus cortex, narrowing of the marginal zones of spleen lymphoid follicles, widening of their germinal centers, and a decrease in the absolute number of cytotoxic T-lymphocytes, NK cells, and B-lymphocytes. After 24 hours of LPS injection the tolerant-to-hypoxia animals had a greater absolute number of T-lymphocytes and NK cells in comparison with the susceptible rats. Thus, in animals with different resistance to hypoxia the LPS-induced SIRS is characterized by different dynamics of morphological and functional changes of the thymus and spleen. The obtained data will serve as a basis for the development of new individual approaches to the prevention and treatment of infectious and inflammatory diseases.
Figures



References
-
- Ghosh D., Kumar R., Pal K. Individual variation in response to simulated hypoxic stress of rats. Indian Journal of Experimental Biology. 2012;50:744–748. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

