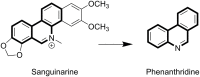
Synthesis and Anticancer Activity Evaluation of Novel Phenanthridine Derivatives
- PMID: 31058081
- PMCID: PMC6478010
- DOI: 10.3389/fonc.2019.00274
Synthesis and Anticancer Activity Evaluation of Novel Phenanthridine Derivatives
Abstract
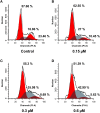
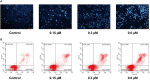
Based on the structure of sanguinarine, fourteen phenanthridine derivatives were designed and synthesized in the current study. The cytotoxic activities of synthesized compounds were evaluated against five human cancer cell lines (MCF-7, PC3, Hela, A549, and HepG2 cell lines) via MTT assay. Among all the compounds tested, molecule 8a exhibited significant cytotoxic activity against MCF-7 cells with a IC50 value of 0.28 μM. A following up enzymatic assay indicated that compound 8a could inhibit the activity of DNA topoisomerase I/II. Further mechanistic studies performed in the MCF-7 cell line revealed that compound 8a could arrest cell cycle in S phase and induce cell apoptosis via downregulation of Bcl-2 and upregulation of Bax. Collectively, a potent DNA topoisomerase inhibitor (8a) was discovered, which exhibited potential as a candidate chemotherapeutic agent for the management of tumors in the present study.
Keywords: anticancer; apoptosis; cell cycle arrest; phenanthridine; topoisomerase.
Figures

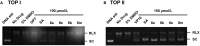
References
LinkOut - more resources
Full Text Sources
Research Materials

