Expression of the Neuroblastoma-Associated ALK-F1174L Activating Mutation During Embryogenesis Impairs the Differentiation of Neural Crest Progenitors in Sympathetic Ganglia
- PMID: 31058082
- PMCID: PMC6477091
- DOI: 10.3389/fonc.2019.00275
Expression of the Neuroblastoma-Associated ALK-F1174L Activating Mutation During Embryogenesis Impairs the Differentiation of Neural Crest Progenitors in Sympathetic Ganglia
Abstract
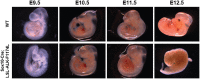
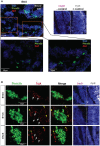
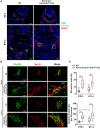
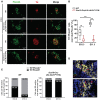
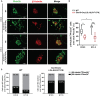
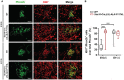
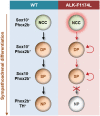
Neuroblastoma (NB) is an embryonal malignancy derived from the abnormal differentiation of the sympathetic nervous system. The Anaplastic Lymphoma Kinase (ALK) gene is frequently altered in NB, through copy number alterations and activating mutations, and represents a predisposition in NB-genesis when mutated. Our previously published data suggested that ALK activating mutations may impair the differentiation potential of neural crest (NC) progenitor cells. Here, we demonstrated that the expression of the endogenous ALK gene starts at E10.5 in the developing sympathetic ganglia (SG). To decipher the impact of deregulated ALK signaling during embryogenesis on the formation and differentiation of sympathetic neuroblasts, Sox10-Cre;LSL-ALK-F1174L embryos were produced to restrict the expression of the human ALK-F1174L transgene to migrating NC cells (NCCs). First, ALK-F1174L mediated an embryonic lethality at mid-gestation and an enlargement of SG with a disorganized architecture in Sox10-Cre;LSL-ALK-F1174L embryos at E10.5 and E11.5. Second, early sympathetic differentiation was severely impaired in Sox10-Cre;LSL-ALK-F1174L embryos. Indeed, their SG displayed a marked increase in the proportion of NCCs and a decrease of sympathetic neuroblasts at both embryonic stages. Third, neuronal and noradrenergic differentiations were blocked in Sox10-Cre;LSL-ALK-F1174L SG, as a reduced proportion of Phox2b+ sympathoblasts expressed βIII-tubulin and almost none were Tyrosine Hydroxylase (TH) positive. Finally, at E10.5, ALK-F1174L mediated an important increase in the proliferation of Phox2b+ progenitors, affecting the transient cell cycle exit observed in normal SG at this embryonic stage. Altogether, we report for the first time that the expression of the human ALK-F1174L mutation in NCCs during embryonic development profoundly disturbs early sympathetic progenitor differentiation, in addition to increasing their proliferation, both mechanisms being potential crucial events in NB oncogenesis.
Keywords: ALK; PHOX2B; SOX10; differentiation; mouse embryos; neural crest cells; neuroblastoma; sympathetic ganglia.
Figures

Similar articles
-
Defining Pathological Activities of ALK in Neuroblastoma, a Neural Crest-Derived Cancer.Int J Mol Sci. 2021 Oct 29;22(21):11718. doi: 10.3390/ijms222111718. Int J Mol Sci. 2021. PMID: 34769149 Free PMC article. Review.
-
Proliferation and Survival of Embryonic Sympathetic Neuroblasts by MYCN and Activated ALK Signaling.J Neurosci. 2016 Oct 5;36(40):10425-10439. doi: 10.1523/JNEUROSCI.0183-16.2016. J Neurosci. 2016. PMID: 27707976 Free PMC article.
-
Wild-type ALK and activating ALK-R1275Q and ALK-F1174L mutations upregulate Myc and initiate tumor formation in murine neural crest progenitor cells.Oncotarget. 2014 Jun 30;5(12):4452-66. doi: 10.18632/oncotarget.2036. Oncotarget. 2014. PMID: 24947326 Free PMC article.
-
Generation of conditional ALK F1174L mutant mouse models for the study of neuroblastoma pathogenesis.Genesis. 2019 Oct;57(10):e23323. doi: 10.1002/dvg.23323. Epub 2019 Jun 20. Genesis. 2019. PMID: 31218818
-
Neuroblastoma models for insights into tumorigenesis and new therapies.Expert Opin Drug Discov. 2015 Jan;10(1):53-62. doi: 10.1517/17460441.2015.974544. Epub 2014 Oct 25. Expert Opin Drug Discov. 2015. PMID: 25345447 Review.
Cited by
-
Application of the FISH method and high-density SNP arrays to assess genetic changes in neuroblastoma-research by one institute.Acta Biochim Pol. 2024 Jul 10;71:12821. doi: 10.3389/abp.2024.12821. eCollection 2024. Acta Biochim Pol. 2024. PMID: 39049899 Free PMC article.
-
Defining Pathological Activities of ALK in Neuroblastoma, a Neural Crest-Derived Cancer.Int J Mol Sci. 2021 Oct 29;22(21):11718. doi: 10.3390/ijms222111718. Int J Mol Sci. 2021. PMID: 34769149 Free PMC article. Review.
-
Lineage-restricted sympathoadrenal progenitors confer neuroblastoma origin and its tumorigenicity.Oncotarget. 2020 Jun 16;11(24):2357-2371. doi: 10.18632/oncotarget.27636. eCollection 2020 Jun 16. Oncotarget. 2020. PMID: 32595833 Free PMC article.
-
Circular RNA DGKB Promotes the Progression of Neuroblastoma by Targeting miR-873/GLI1 Axis.Front Oncol. 2020 Jul 23;10:1104. doi: 10.3389/fonc.2020.01104. eCollection 2020. Front Oncol. 2020. PMID: 32793474 Free PMC article.
-
Multifocal Neuroblastoma and Central Hypoventilation in An Infant with Germline ALK F1174I Mutation.Diagnostics (Basel). 2022 Sep 19;12(9):2260. doi: 10.3390/diagnostics12092260. Diagnostics (Basel). 2022. PMID: 36140661 Free PMC article.
References
-
- Vo KT, Matthay KK, Neuhaus J, London WB, Hero B, Ambros PF, et al. . Clinical, biologic, and prognostic differences on the basis of primary tumor site in neuroblastoma: a report from the international neuroblastoma risk group project. J Clin Oncol. (2014) 32:3169–76. 10.1200/jco.2014.56.1621 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

