Transcriptional Regulation of Ovarian Steroidogenic Genes: Recent Findings Obtained from Stem Cell-Derived Steroidogenic Cells
- PMID: 31058195
- PMCID: PMC6463655
- DOI: 10.1155/2019/8973076
Transcriptional Regulation of Ovarian Steroidogenic Genes: Recent Findings Obtained from Stem Cell-Derived Steroidogenic Cells
Abstract
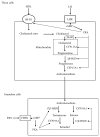
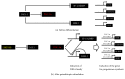
Ovaries represent one of the primary steroidogenic organs, producing estrogen and progesterone under the regulation of gonadotropins during the estrous cycle. Gonadotropins fluctuate the expression of various steroidogenesis-related genes, such as those encoding steroidogenic enzymes, cholesterol deliverer, and electronic transporter. Steroidogenic factor-1 (SF-1)/adrenal 4-binding protein (Ad4BP)/NR5A1 and liver receptor homolog-1 (LRH-1) play important roles in these phenomena via transcriptional regulation. With the aid of cAMP, SF-1/Ad4BP and LRH-1 can induce the differentiation of stem cells into steroidogenic cells. This model is a useful tool for studying the molecular mechanisms of steroidogenesis. In this article, we will provide insight into the transcriptional regulation of steroidogenesis-related genes in ovaries that are revealed from stem cell-derived steroidogenic cells. Using the cells derived from the model, novel SF-1/Ad4BP- and LRH-1-regulated genes were identified by combined DNA microarray and promoter tiling array analyses. The interaction of SF-1/Ad4BP and LRH-1 with transcriptional regulators in the regulation of ovarian steroidogenesis was also revealed.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

