Apolipoprotein E associated with reconstituted high-density lipoprotein-like particles is protected from aggregation
- PMID: 31058310
- PMCID: PMC6617784
- DOI: 10.1002/1873-3468.13428
Apolipoprotein E associated with reconstituted high-density lipoprotein-like particles is protected from aggregation
Abstract
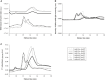
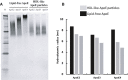
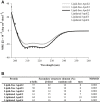
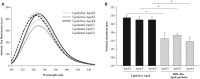
Apolipoprotein E (APOE) genotype determines Alzheimer's disease (AD) susceptibility, with the APOE ε4 allele being an established risk factor for late-onset AD. The ApoE lipidation status has been reported to impact amyloid-beta (Aβ) peptide metabolism. The details of how lipidation affects ApoE behavior remain to be elucidated. In this study, we prepared lipid-free and lipid-bound ApoE particles, mimicking the high-density lipoprotein particles found in vivo, for all three isoforms (ApoE2, ApoE3, and ApoE4) and biophysically characterized them. We find that lipid-free ApoE in solution has the tendency to aggregate in vitro in an isoform-dependent manner under near-physiological conditions and that aggregation is impeded by lipidation of ApoE.
Keywords: Alzheimer's disease; aggregation; apolipoprotein E; high-density lipoprotein; isoform; lipidation.
© 2019 The Authors. FEBS Letters published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Figures


References
-
- Mahley RW (1988) Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 240, 622–630. - PubMed
-
- Zannis VI and Breslow JL (1981) Human very low density lipoprotein apolipoprotein E isoprotein polymorphism is explained by genetic variation and posttranslational modification. Biochemistry 20, 1033–1041. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

