Functional connectivity along the anterior-posterior axis of hippocampal subfields in the ageing human brain
- PMID: 31058404
- PMCID: PMC6849752
- DOI: 10.1002/hipo.23097
Functional connectivity along the anterior-posterior axis of hippocampal subfields in the ageing human brain
Erratum in
-
Erratum.Hippocampus. 2020 Aug;30(8):909. doi: 10.1002/hipo.23249. Hippocampus. 2020. PMID: 32812312 Free PMC article. No abstract available.
Abstract
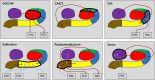
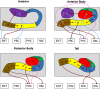
While age-related volumetric changes in human hippocampal subfields have been reported, little is known about patterns of subfield functional connectivity (FC) in the context of healthy ageing. Here we investigated age-related changes in patterns of FC down the anterior-posterior axis of each subfield. Using high resolution structural MRI we delineated the dentate gyrus (DG), CA fields (including separating DG from CA3), the subiculum, pre/parasubiculum, and the uncus in healthy young and older adults. We then used high resolution resting state functional MRI to measure FC in each group and to directly compare them. We first examined the FC of each subfield in its entirety, in terms of FC with other subfields and with neighboring cortical regions, namely, entorhinal, perirhinal, posterior parahippocampal, and retrosplenial cortices. Next, we analyzed subfield to subfield FC within different portions along the hippocampal anterior-posterior axis, and FC of each subfield portion with the neighboring cortical regions of interest. In general, the FC of the older adults was similar to that observed in the younger adults. We found that, as in the young group, the older group displayed intrinsic FC between the subfields that aligned with the tri-synaptic circuit but also extended beyond it, and that FC between the subfields and neighboring cortical areas differed markedly along the anterior-posterior axis of each subfield. We observed only one significant difference between the young and older groups. Compared to the young group, the older participants had significantly reduced FC between the anterior CA1-subiculum transition region and the transentorhinal cortex, two brain regions known to be disproportionately affected during the early stages of age-related tau accumulation. Overall, these results contribute to ongoing efforts to characterize human hippocampal subfield connectivity, with implications for understanding hippocampal function and its modulation in the ageing brain.
Keywords: ageing; functional connectivity; hippocampal subfields; perirhinal cortex; subiculum; tau.
© 2019 The Authors. Hippocampus published by Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Augustinack, J. C. , Huber, K. E. , Stevens, A. A. , Roy, M. , Frosch, M. P. , van der Kouwe, A. J. , … the Alzheimer's Disease Neuroimaging Initiative . (2013). Predicting the location of human perirhinal cortex, Brodmann's area 35, from MRI. NeuroImage, 64, 32–42. 10.1016/j.neuroimage.2012.08.071 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

