Three-Component Protein Modification Using Mercaptobenzaldehyde Derivatives
- PMID: 31058515
- PMCID: PMC6767916
- DOI: 10.1021/acs.orglett.9b01294
Three-Component Protein Modification Using Mercaptobenzaldehyde Derivatives
Abstract
A chemoselective primary amine modification strategy that enables the three-component, one-pot bioconjugation is described. The specifically designed, mercaptobenzaldehyde-based bifunctional linker achieves highly selective and robust amine labeling under biocompatible conditions. This linker demonstrates wide functional group tolerance and is simple to prepare, which allowed facile payload incorporation. Finally, our studies have shown that the introduction of linker does not impair the function of modified protein such as insulin.
Figures
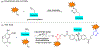



References
-
- For selected reviews: (a) deGruyter JN; Malins LR; Baran PS Residue-Specific Peptide Modification: A Chemist's Guide. Biochemistry, 2017, 56, 3863–3873 - PMC - PubMed
- Tamura T; Hamachi I Chemistry for Covalent Modification of Endogenous/Native Proteins: From Test Tubes to Complex Biological Systems. J. Am. Chem. Soc 2019, 141, 2782–2799 - PubMed
- Spicer CD; Davis BG Selective chemical protein modification. Nat. Commun 2014, 5, 4740–4754 - PubMed
- Lang K; Chin JW Bioorthogonal Reactions for Labeling Proteins. ACS Chem. Biol 2014, 9,16–20 - PubMed
- Sletten EM; Bertozzi CR Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality. Angew. Chem., Int. Ed 2009, 48, 6974–6998 - PMC - PubMed
- Krall N; Da Cruz FP; Boutureira O; Bernardes GJ Site-selective protein-modification chemistry for basic biology and drug development. L. Nat. Chem 2016, 8, 103–113 - PubMed
- Omar Boutureira O; Bernardes GJ Advances in Chemical Protein Modification. Chem. Rev 2015, 115, 2174–2195 - PubMed
- Silvestri AP; Cistrone PA; Dawson PE Adapting the Glaser Reaction for Bioconjugation: Robust Access to Structurally Simple, Rigid Linkers. Angew. Chem. Int. Ed 2017, 56, 10438–10442 - PMC - PubMed
- Devaraj NK The Future of Bioorthogonal Chemistry. ACS Cent. Sci, 2018, 4, 952–959 - PMC - PubMed
- Reguera L; Yanira Méndez Y; Humpierre AR; Valdés O; Rivera DG Multicomponent Reactions in Ligation and Bioconjugation Chemistry. Acc. Chem. Res, 2018, 51, 1475–1486 - PubMed
- Li J; Chen PR Development and application of bond cleavage reactions in bioorthogonal chemistry. Nat. Chem. Biol 2016, 12, 129–137 - PubMed
- Konievab O; Wagner A Developments and recent advancements in the field of endogenous amino acid selective bond forming reactions for bioconjugation. Chem. Soc. Rev, 2015, 44, 5495–5551 - PubMed
- Blagg J; Workman P Choose and Use Your Chemical Probe Wisely to Explore Cancer Biology. Cancer Cell 2017, 32, 9–25. - PMC - PubMed
-
- Fisher SA; Baker AEG; Shoichet MS Designing Peptide and Protein Modified Hydrogels: Selecting the Optimal Conjugation Strategy. J. Am. Chem. Soc 2017, 139, 7416–7427. - PubMed
-
- Wright MH; Sieber SA Chemical proteomics approaches for identifying the cellular targets of natural products. Nat. Prod. Rep, 2016, 33, 681–708 - PMC - PubMed
- Leitner A Cross-linking and other structural proteomics techniques: how chemistry is enabling mass spectrometry applications in structural biology. Chem. Sci, 2016, 7, 4792–4803 - PMC - PubMed
- MacKinnon AL; Taunton J Target Identification by Diazirine Photo-Cross-linking and Click Chemistry. Curr. Protoc. Chem. Biol 2009, 1, 55–73 - PMC - PubMed
- Carrico IS Chemoselective modification of proteins: hitting the target. Chem. Soc. Rev, 2008, 37, 1423–1431. - PubMed
-
- Mu J; Liu F; Shafiq Rajab M; Shi M; Li S; Goh C; Lu L; Xu Q; Liu B; Ng L; Xing B-G A Small-Molecule FRET Reporter for the Real-Time Visualization of Cell-Surface Proteolytic Enzyme Functions. Angew. Chem. Int. Ed 2014, 53, 14357–14362 - PubMed
- Weinstain R; Savariar EN; Felsen CN; Tsien RY In Vivo Targeting of Hydrogen Peroxide by Activatable Cell-Penetrating Peptides. J. Am. Chem. Soc 2014, 136, 874–877. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

