Fibroblasts as Modulators of Local and Systemic Cancer Metabolism
- PMID: 31058816
- PMCID: PMC6562905
- DOI: 10.3390/cancers11050619
Fibroblasts as Modulators of Local and Systemic Cancer Metabolism
Abstract
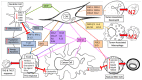
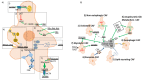
Fibroblast activation is an accompanying feature of solid tumor progression, resembling a conserved host response to tissue damage. Cancer-associated fibroblasts (CAFs) comprise a heterogeneous and plastic population with increasingly appreciated roles in tumor growth, metastatic capacity, and response to therapy. Classical features of fibroblasts in a wound-healing response, including profound extracellular matrix production and cytokine release, are recapitulated in cancer. Emerging evidence suggests that fibroblastic cells in the microenvironments of solid tumors also critically modulate cellular metabolism in the neoplastic compartment through mechanisms including paracrine transfer of metabolites or non-cell-autonomous regulation of metabolic signaling pathways. These metabolic functions may represent common mechanisms by which fibroblasts stimulate growth of the regenerating epithelium during a wound-healing reaction, or may reflect unique co-evolution of cancer cells and surrounding stroma within the tumor microenvironment. Here we review the recent literature supporting an important role for CAFs in regulation of cancer cell metabolism, and relevant pathways that may serve as targets for therapeutic intervention.
Keywords: cancer metabolism; cancer-associated fibroblast; tumor-stroma crosstalk.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Paunescu V., Bojin F.M., Tatu C.A., Gavriliuc O.I., Rosca A., Gruia A.T., Tanasie G., Bunu C., Crisnic D., Gherghiceanu M., et al. Tumour-associated fibroblasts and mesenchymal stem cells: More similarities than differences. J. Cell. Mol. Med. 2011;15:635–646. doi: 10.1111/j.1582-4934.2010.01044.x. - DOI - PMC - PubMed
Publication types
Grants and funding
- CA188259/National Cancer Institute
- CA229580/National Cancer Institute
- 132898-RSG-18-142-01-CSM/American Cancer Society
- V Scholar Award/V Foundation for Cancer Research
- W81XWH-18-1-0437/DOD Peer Reviewed Cancer Research Program
- Seed Grant/Hirshberg Foundation for Pancreatic Cancer Research
- New Investigator Grant/Medical Research Foundation
- Brenden-Colson Center Pilot Award/Oregon Health and Science University
- Cancer Early Detection Advanced Research Pilot Award/Oregon Health and Science University
- Fellowship for Diversity in Research/Oregon Health and Science University
LinkOut - more resources
Full Text Sources

