Association of Blood and Cerebrospinal Fluid Tau Level and Other Biomarkers With Survival Time in Sporadic Creutzfeldt-Jakob Disease
- PMID: 31058916
- PMCID: PMC6503575
- DOI: 10.1001/jamaneurol.2019.1071
Association of Blood and Cerebrospinal Fluid Tau Level and Other Biomarkers With Survival Time in Sporadic Creutzfeldt-Jakob Disease
Abstract
Importance: Fluid biomarkers that can predict survival time in sporadic Creutzfeldt-Jakob disease (sCJD) will be critical for clinical care and for treatment trials.
Objective: To assess whether plasma and cerebrospinal fluid (CSF) biomarkers are associated with survival time in patients with sCJD.
Design, setting, and participants: In this longitudinal cohort study, data from 193 patients with probable or definite sCJD who had codon 129 genotyping referred to a tertiary national referral service in the United States were collected from March 2004 to January 2018. Participants were evaluated until death or censored at the time of statistical analysis (range, 0.03-38.3 months). We fitted Cox proportional hazard models with time to event as the outcome. Fluid biomarkers were log-transformed, and models were run with and without nonfluid biomarkers of survival. Five patients were excluded because life-extending measures were performed.
Main outcomes and measures: Biomarkers of survival included sex, age, codon 129 genotype, Barthel Index, Medical Research Council Prion Disease Rating Scale, 8 CSF biomarkers (total tau [t-tau] level, phosphorylated tau [p-tau] level, t-tau:p-tau ratio, neurofilament light [NfL] level, β-amyloid 42 level, neuron-specific enolase level, 14-3-3 test result, and real-time quaking-induced conversion test), and 3 plasma biomarkers (t-tau level, NfL level, and glial fibrillary acidic protein level).
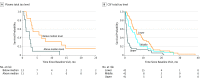
Results: Of the 188 included participants, 103 (54.8%) were male, and the mean (SD) age was 63.8 (9.2) years. Plasma t-tau levels (hazard ratio, 5.8; 95% CI, 2.3-14.8; P < .001) and CSF t-tau levels (hazard ratio, 1.6; 95% CI, 1.2-2.1; P < .001) were significantly associated with survival after controlling for codon 129 genotype and Barthel Index, which are also associated with survival time. Plasma and CSF t-tau levels were correlated (r = 0.74; 95% CI, 0.50-0.90; P < .001). Other fluid biomarkers associated with survival included plasma NfL levels, CSF NfL levels, t-tau:p-tau ratio, 14-3-3 test result, and neuron-specific enolase levels. In a restricted subset of 23 patients with data for all significant biomarkers, the hazard ratio for plasma t-tau level was more than 40% larger than any other biomarkers (hazard ratio, 3.4; 95% CI, 1.8-6.4).
Conclusions and relevance: Cerebrospinal fluid and plasma tau levels, along with several other fluid biomarkers, were significantly associated with survival time in patients with sCJD. The correlation between CSF and plasma t-tau levels and the association of plasma t-tau level with survival time suggest that plasma t-tau level may be a minimally invasive fluid biomarker in sCJD that could improve clinical trial stratification and guide clinical care.
Conflict of interest statement
Figures