Effect of Sunscreen Application Under Maximal Use Conditions on Plasma Concentration of Sunscreen Active Ingredients: A Randomized Clinical Trial
- PMID: 31058986
- PMCID: PMC6549296
- DOI: 10.1001/jama.2019.5586
Effect of Sunscreen Application Under Maximal Use Conditions on Plasma Concentration of Sunscreen Active Ingredients: A Randomized Clinical Trial
Abstract
Importance: The US Food and Drug Administration (FDA) has provided guidance that sunscreen active ingredients with systemic absorption greater than 0.5 ng/mL or with safety concerns should undergo nonclinical toxicology assessment including systemic carcinogenicity and additional developmental and reproductive studies.
Objective: To determine whether the active ingredients (avobenzone, oxybenzone, octocrylene, and ecamsule) of 4 commercially available sunscreens are absorbed into systemic circulation.
Design, setting, and participants: Randomized clinical trial conducted at a phase 1 clinical pharmacology unit in the United States and enrolling 24 healthy volunteers. Enrollment started in July 2018 and ended in August 2018.
Interventions: Participants were randomized to 1 of 4 sunscreens: spray 1 (n = 6 participants), spray 2 (n = 6), a lotion (n = 6), and a cream (n = 6). Two milligrams of sunscreen per 1 cm2 was applied to 75% of body surface area 4 times per day for 4 days, and 30 blood samples were collected over 7 days from each participant.
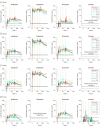
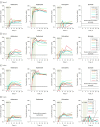
Main outcomes and measures: The primary outcome was the maximum plasma concentration of avobenzone. Secondary outcomes were the maximum plasma concentrations of oxybenzone, octocrylene, and ecamsule.
Results: Among 24 participants randomized (mean age, 35.5 [SD, 1.5] years; 12 (50%] women; 14 [58%] black or African American; 14 [58%]), 23 (96%) completed the trial. For avobenzone, geometric mean maximum plasma concentrations were 4.0 ng/mL (coefficient of variation, 6.9%) for spray 1; 3.4 ng/mL (coefficient of variation, 77.3%) for spray 2; 4.3 ng/mL (coefficient of variation, 46.1%) for lotion; and 1.8 ng/mL (coefficient of variation, 32.1%). For oxybenzone, the corresponding values were 209.6 ng/mL (66.8%) for spray 1, 194.9 ng/mL (52.4%) for spray 2, and 169.3 ng/mL (44.5%) for lotion; for octocrylene, 2.9 ng/mL (102%) for spray 1, 7.8 ng/mL (113.3%) for spray 2, 5.7 ng/mL (66.3%) for lotion, and 5.7 ng/mL (47.1%) for cream; and for ecamsule, 1.5 ng/mL (166.1%) for cream. Systemic concentrations greater than 0.5 ng/mL were reached for all 4 products after 4 applications on day 1. The most common adverse event was rash, which developed in 1 participant with each sunscreen.
Conclusions and relevance: In this preliminary study involving healthy volunteers, application of 4 commercially available sunscreens under maximal use conditions resulted in plasma concentrations that exceeded the threshold established by the FDA for potentially waiving some nonclinical toxicology studies for sunscreens. The systemic absorption of sunscreen ingredients supports the need for further studies to determine the clinical significance of these findings. These results do not indicate that individuals should refrain from the use of sunscreen.
Trial registration: ClinicalTrials.gov Identifier: NCT03582215.
Conflict of interest statement
Figures

Comment in
-
Filling in the Evidence About Sunscreen.JAMA. 2019 Jun 4;321(21):2077-2079. doi: 10.1001/jama.2019.5528. JAMA. 2019. PMID: 31058950 No abstract available.
-
Effect of sunscreen application under maximal-use conditions on plasma concentration of sunscreen active ingredients: a critical appraisal.Br J Dermatol. 2020 Jun;182(6):1345-1347. doi: 10.1111/bjd.18803. Epub 2020 Feb 3. Br J Dermatol. 2020. PMID: 31834937 No abstract available.
References
-
- US Food and Drug Administration (FDA) Guidance for Industry: Nonprescription Sunscreen Drug Products—Safety and Effectiveness Data. FDA website. https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformat.... Published November 2016. Accessed February 5, 2019.
-
- US Food and Drug Administration, HHS Labeling and effectiveness testing: sunscreen drug products for over-the-counter human use: final rule. Fed Regist. 2011;76(117):35620-35665. - PubMed
-
- 21 CFR §700.35: cosmetics containing sunscreen ingredients. US Government Publishing Office website. https://www.govinfo.gov/content/pkg/CFR-2011-title21-vol7/xml/CFR-2011-t.... 2011. Accessed February 5, 2019.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

