Molecular profile in Paraguayan colorectal cancer patients, towards to a precision medicine strategy
- PMID: 31059199
- PMCID: PMC6558499
- DOI: 10.1002/cam4.2191
Molecular profile in Paraguayan colorectal cancer patients, towards to a precision medicine strategy
Abstract
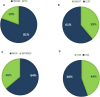
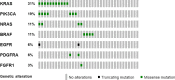
Somatic mutation analysis and evaluation of microsatellite instability (MSI) have become mandatory for selecting personalized therapy strategies for advanced colorectal cancer and are not available as routine methods in Paraguay. The aims of this study were to analyze the molecular profile as well as the microsatellite status in a series of advanced colorectal patients from two public hospitals from Paraguay, to introduce these methodologies in the routine practice to guide the therapeutic decisions. Thirty-six patients diagnosed with advanced colorectal cancer from two referent public hospitals from Paraguay were recruited from May 2017 to February 2018. Sequenom Mass spectrometry, Oncocarta Panel V.1 was applied to analyze the mutational profile from formalin-fixed paraffin-embedded samples. The microsatellite status was tested by immunohistochemistry (IHC). The mean age of the patients was 52 years with a range from 20 to 74 years. Eighty-three percent of the patients included in the study have advanced-stage tumors at the moment of the diagnosis. Sixteen patients (44.4%) were wild-type for all the oncogene regions analyzed with the Oncocarta panel. Thirty-two hot-spot pathogenic variants on seven oncogenes, among 20 patients (55.6%), were identified, including KRAS, NRAS, BRAF, PI3KCA, FGFR, epidermal growth factor receptor, and PDGFRA. Moreover, 14 (38.8%) of these patients presented pathogenic variants in KRAS/NRAS or BRAF genes that have implications in the clinical practice decisions. Five patients (14%) presented MSI. The IHC study for microsatellite status and the molecular profile analysis through Sequenom mass spectrometry are feasible and useful methods, due to identify those patient candidates for targeted therapies and for the budgetary calculations of the National Health Plans.
Keywords: Oncocarta; colorectal cancer; microsatellite instability; mutational profile; precision medicine.
© 2019 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures



References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. - PubMed
-
- Bray F, Pineros M. Cancer patterns, trends and projections in Latin America and the Caribbean: a global context. Salud Publica Mex. 2016;58:104‐117. - PubMed
-
- Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first‐line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open‐label, phase 3 TRIBE study. Lancet Oncol. 2015;16:1306‐1315. - PubMed
-
- Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first‐line treatment for patients with metastatic colorectal cancer (FIRE‐3): a randomised, open‐label, phase 3 trial. Lancet Oncol. 2014;15:1065‐1075. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

