Onset of Telomere Dysfunction and Fusions in Human Ovarian Carcinoma
- PMID: 31060240
- PMCID: PMC6562548
- DOI: 10.3390/cells8050414
Onset of Telomere Dysfunction and Fusions in Human Ovarian Carcinoma
Abstract
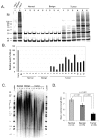
Telomere dysfunction has been strongly implicated in the initiation of genomic instability and is suspected to be an early event in the carcinogenesis of human solid tumors. Recent findings have established the presence of telomere fusions in human breast and prostate malignancies; however, the onset of this genomic instability mechanism during progression of other solid cancers is not well understood. Herein, we explored telomere dynamics in patient-derived epithelial ovarian cancers (OC), a malignancy characterized by multiple distinct subtypes, extensive molecular heterogeneity, and widespread genomic instability. We discovered a high frequency of telomere fusions in ovarian tumor tissues; however, limited telomere fusions were detected in normal adjacent tissues or benign ovarian samples. In addition, we found relatively high levels of both telomerase activity and hTERT expression, along with anaphase bridges in tumor tissues, which were notably absent in adjacent normal ovarian tissues and benign lesions. These results suggest that telomere dysfunction may occur early in ovarian carcinogenesis and, importantly, that it may play a critical role in the initiation and progression of the disease. Recognizing telomere dysfunction as a pervasive feature of this heterogeneous malignancy may facilitate the future development of novel diagnostic tools and improved methods of disease monitoring and treatment.
Keywords: genomic instability; ovarian carcinoma; telomerase; telomere; telomere dysfunction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

