Mesenchymal Stem Cell-Conditioned Medium Induces Neutrophil Apoptosis Associated with Inhibition of the NF-κB Pathway in Endotoxin-Induced Acute Lung Injury
- PMID: 31060326
- PMCID: PMC6540353
- DOI: 10.3390/ijms20092208
Mesenchymal Stem Cell-Conditioned Medium Induces Neutrophil Apoptosis Associated with Inhibition of the NF-κB Pathway in Endotoxin-Induced Acute Lung Injury
Abstract
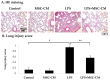
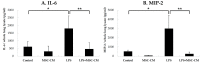
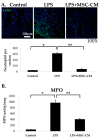
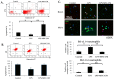
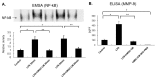
The immunomodulatory effects of mesenchymal stem cells (MSCs) are established. However, the effects of MSCs on neutrophil survival in acute lung injury (ALI) remain unclear. The goal of this study was to investigate the effect of an MSC-conditioned medium (MSC-CM) on neutrophil apoptosis in endotoxin-induced ALI. In this study, an MSC-CM was delivered via tail vein injection to wild-type male C57BL/6 mice 4 h after an intratracheal injection of lipopolysaccharide (LPS). Twenty-four hours later, bronchoalveolar lavage fluid (BALF) and lung tissue were collected to perform histology, immunohistochemistry, apoptosis assay of neutrophil, enzyme-linked immunosorbent assays, and an electrophoretic mobility shift assay. Human neutrophils were also collected from patients with sepsis-induced acute respiratory distress syndrome (ARDS). Human neutrophils were treated in vitro with LPS, with or without subsequent MSC-CM co-treatment, and were then analyzed. Administration of the MSC-CM resulted in a significant attenuation of histopathological changes, the levels of interleukin-6 and macrophage inflammatory protein 2, and neutrophil accumulation in mouse lung tissues of LPS-induced ALI. Additionally, MSC-CM therapy enhanced the apoptosis of BALF neutrophils and reduced the expression of the anti-apoptotic molecules, Bcl-xL and Mcl-1, both in vivo and in vitro experiments. Furthermore, phosphorylated and total levels of nuclear factor (NF)-κB p65 were reduced in lung tissues from LPS + MSC-CM mice. Human MSC-CM also reduced the activity levels of NF-κB and matrix metalloproteinase-9 in the human neutrophils from ARDS patients. Thus, the results of this study suggest that the MSC-CM attenuated LPS-induced ALI by inducing neutrophil apoptosis, associated with inhibition of the NF-κB pathway.
Keywords: Mesenchymal Stem Cell; Neutrophil apoptosis; acute lung injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kangelaris K.N., Prakash A., Liu K.D., Aouizerat B., Woodruff P.G., Erle D.J., Rogers A., Seeley E.J., Chu J., Liu T., et al. Increased expression of neutrophil-related genes in patients with early sepsis-induced ards. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015;308:L1102–L1113. doi: 10.1152/ajplung.00380.2014. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

