The Immune Microenvironment in Hormone Receptor-Positive Breast Cancer Before and After Preoperative Chemotherapy
- PMID: 31061067
- PMCID: PMC6677598
- DOI: 10.1158/1078-0432.CCR-19-0173
The Immune Microenvironment in Hormone Receptor-Positive Breast Cancer Before and After Preoperative Chemotherapy
Abstract
Purpose: Hormone receptor-positive/HER2-negative (HR+/HER2-) breast cancer is associated with low levels of stromal tumor-infiltrating lymphocytes (sTIL) and PD-L1, and demonstrates poor responses to checkpoint inhibitor therapy. Evaluating the effect of standard chemotherapy on the immune microenvironment may suggest new opportunities for immunotherapy-based approaches to treating HR+/HER2- breast tumors.
Experimental design: HR+/HER2- breast tumors were analyzed before and after neoadjuvant chemotherapy. sTIL were assessed histologically; CD8+ cells, CD68+ cells, and PD-L1 staining were assessed immunohistochemically; whole transcriptome sequencing and panel RNA expression analysis (NanoString) were performed.
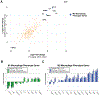
Results: Ninety-six patients were analyzed from two cohorts (n = 55, Dana-Farber cohort; n = 41, MD Anderson cohort). sTIL, CD8, and PD-L1 on tumor cells were higher in tumors with basal PAM50 intrinsic subtype. Higher levels of tissue-based lymphocyte (sTIL, CD8, PD-L1) and macrophage (CD68) markers, as well as gene expression markers of lymphocyte or macrophage phenotypes (NanoString or CIBERSORT), correlated with favorable response to neoadjuvant chemotherapy, but not with improved distant metastasis-free survival in these cohorts or a large gene expression dataset (N = 302). In paired pre-/postchemotherapy samples, sTIL and CD8+ cells were significantly decreased after treatment, whereas expression analyses (NanoString) demonstrated significant increase of multiple myeloid signatures. Single gene expression implicated increased expression of immunosuppressive (M2-like) macrophage-specific genes after chemotherapy.
Conclusions: The immune microenvironment of HR+/HER2- tumors differs according to tumor biology. This cohort of paired pre-/postchemotherapy samples suggests a critical role for immunosuppressive macrophage expansion in residual disease. The role of macrophages in chemoresistance should be explored, and further evaluation of macrophage-targeting therapy is warranted.
©2019 American Association for Cancer Research.
Figures




References
-
- Issa-Nummer Y, Darb-Esfahani S, Loibl S, Kunz G, Nekljudova V, Schrader I, et al. Prospective validation of immunological infiltrate for prediction of response to neoadjuvant chemotherapy in HER2−negative breast cancer--a substudy of the neoadjuvant GeparQuinto trial. PloS one 2013;8(12):e79775 doi 10.1371/journal.pone.0079775. - DOI - PMC - PubMed
-
- Vidula N, Yau C, Goga A, Rugo HS. Programmed cell death 1 (PD-1) receptor and prograamed death ligand 1 (PD-L1) expression in primary breast cancer (BC); correlations with clinical characteristics and patient outcomes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015;33:suppl; abstr 1090.
-
- Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia 2006;8(3):190–8 doi 10.1593/neo.05733. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

