PMP22 Regulates Cholesterol Trafficking and ABCA1-Mediated Cholesterol Efflux
- PMID: 31061090
- PMCID: PMC6607759
- DOI: 10.1523/JNEUROSCI.2942-18.2019
PMP22 Regulates Cholesterol Trafficking and ABCA1-Mediated Cholesterol Efflux
Abstract
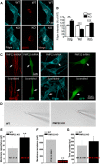
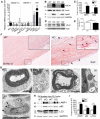
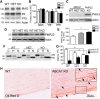
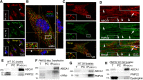
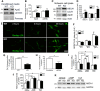
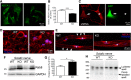
The absence of functional peripheral myelin protein 22 (PMP22) is associated with shortened lifespan in rodents and severe peripheral nerve myelin abnormalities in several species including humans. Schwann cells and nerves from PMP22 knock-out (KO) mice show deranged cholesterol distribution and aberrant lipid raft morphology, supporting an unrecognized role for PMP22 in cellular lipid metabolism. To examine the mechanisms underlying these abnormalities, we studied Schwann cells and nerves from male and female PMP22 KO mice. Whole-cell current-clamp recordings in cultured Schwann cells revealed increased membrane capacitance and decreased membrane resistance in the absence of PMP22, which was consistent with a reduction in membrane cholesterol. Nerves from PMP22-deficient mice contained abnormal lipid droplets, with both mRNA and protein levels of apolipoprotein E (apoE) and ATP-binding cassette transporter A1 (ABCA1) being highly upregulated. Despite the upregulation of ABCA1 and apoE, the absence of PMP22 resulted in reduced localization of the transporter to the cell membrane and diminished secretion of apoE. The absence of PMP22 also impaired ABCA1-mediated cholesterol efflux capacity. In nerves from ABCA1 KO mice, the expression of PMP22 was significantly elevated and the subcellular processing of the overproduced protein was aberrant. In wild-type samples, double immunolabeling identified overlapping distribution of PMP22 and ABCA1 at the Schwann cell plasma membrane and the two proteins were coimmunoprecipitated from Schwann cell and nerve lysates. Together, these results reveal a novel role for PMP22 in regulating lipid metabolism and cholesterol trafficking through functional interaction with the cholesterol efflux regulatory protein ABCA1.SIGNIFICANCE STATEMENT Understanding the subcellular events that underlie abnormal myelin formation in hereditary neuropathies is critical for advancing therapy development. Peripheral myelin protein 22 (PMP22) is an essential peripheral myelin protein because its genetic abnormalities account for ∼80% of hereditary neuropathies. Here, we demonstrate that in the absence of PMP22, the cellular and electrophysiological properties of the Schwann cells' plasma membrane are altered and cholesterol trafficking and lipid homeostasis are perturbed. The molecular mechanisms for these abnormalities involve a functional interplay among PMP22, cholesterol, apolipoprotein E, and the major cholesterol-efflux transporter protein ATP-binding cassette transporter A1 (ABCA1). These findings establish a critical role for PMP22 in the maintenance of cholesterol homeostasis in Schwann cells.
Keywords: ATP-binding cassette transporter 1; Schwann cell; apoE; cholesterol efflux; hereditary neuropathy; peripheral myelin protein 22.
Copyright © 2019 the authors.
Figures

Similar articles
-
Roles for PMP22 in Schwann cell cholesterol homeostasis in health and disease.Biochem Soc Trans. 2024 Aug 28;52(4):1747-1756. doi: 10.1042/BST20231359. Biochem Soc Trans. 2024. PMID: 38979632 Free PMC article. Review.
-
PMP22 is critical for actin-mediated cellular functions and for establishing lipid rafts.J Neurosci. 2014 Nov 26;34(48):16140-52. doi: 10.1523/JNEUROSCI.1908-14.2014. J Neurosci. 2014. PMID: 25429154 Free PMC article.
-
Subcellular diversion of cholesterol by gain- and loss-of-function mutations in PMP22.Glia. 2020 Nov;68(11):2300-2315. doi: 10.1002/glia.23840. Epub 2020 Jun 8. Glia. 2020. PMID: 32511821
-
PMP22 duplication dysregulates lipid homeostasis and plasma membrane organization in developing human Schwann cells.Brain. 2024 Sep 3;147(9):3113-3130. doi: 10.1093/brain/awae158. Brain. 2024. PMID: 38743588 Free PMC article.
-
The target of regulating the ATP-binding cassette A1 protein (ABCA1): promoting ABCA1-mediated cholesterol efflux in different cells.Curr Pharm Biotechnol. 2013;14(6):623-31. doi: 10.2174/138920101131400228. Curr Pharm Biotechnol. 2013. PMID: 24016265 Review.
Cited by
-
Held Up in Traffic-Defects in the Trafficking Machinery in Charcot-Marie-Tooth Disease.Front Mol Neurosci. 2021 Aug 16;14:695294. doi: 10.3389/fnmol.2021.695294. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34483837 Free PMC article. Review.
-
Association of lipid rafts cholesterol with clinical profile in fragile X syndrome.Sci Rep. 2022 Feb 21;12(1):2936. doi: 10.1038/s41598-022-07064-z. Sci Rep. 2022. PMID: 35190617 Free PMC article.
-
Ion mobility-mass spectrometry reveals the role of peripheral myelin protein dimers in peripheral neuropathy.Proc Natl Acad Sci U S A. 2021 Apr 27;118(17):e2015331118. doi: 10.1073/pnas.2015331118. Proc Natl Acad Sci U S A. 2021. PMID: 33893233 Free PMC article.
-
Myelin Defects in Niemann-Pick Type C Disease: Mechanisms and Possible Therapeutic Perspectives.Int J Mol Sci. 2021 Aug 17;22(16):8858. doi: 10.3390/ijms22168858. Int J Mol Sci. 2021. PMID: 34445564 Free PMC article. Review.
-
Mechanisms and treatment strategies of demyelinating and dysmyelinating Charcot-Marie-Tooth disease.Neural Regen Res. 2023 Sep;18(9):1931-1939. doi: 10.4103/1673-5374.367834. Neural Regen Res. 2023. PMID: 36926710 Free PMC article. Review.
References
-
- Amici SA, Dunn WA Jr, Murphy AJ, Adams NC, Gale NW, Valenzuela DM, Yancopoulos GD, Notterpek L (2006) Peripheral myelin protein 22 is in complex with alpha6beta4 integrin, and its absence alters the Schwann cell basal lamina. J Neurosci 26:1179–1189. 10.1523/JNEUROSCI.2618-05.2006 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
