Chemical disarming of isoniazid resistance in Mycobacterium tuberculosis
- PMID: 31061116
- PMCID: PMC6535022
- DOI: 10.1073/pnas.1818009116
Chemical disarming of isoniazid resistance in Mycobacterium tuberculosis
Abstract
Mycobacterium tuberculosis (Mtb) killed more people in 2017 than any other single infectious agent. This dangerous pathogen is able to withstand stresses imposed by the immune system and tolerate exposure to antibiotics, resulting in persistent infection. The global tuberculosis (TB) epidemic has been exacerbated by the emergence of mutant strains of Mtb that are resistant to frontline antibiotics. Thus, both phenotypic drug tolerance and genetic drug resistance are major obstacles to successful TB therapy. Using a chemical approach to identify compounds that block stress and drug tolerance, as opposed to traditional screens for compounds that kill Mtb, we identified a small molecule, C10, that blocks tolerance to oxidative stress, acid stress, and the frontline antibiotic isoniazid (INH). In addition, we found that C10 prevents the selection for INH-resistant mutants and restores INH sensitivity in otherwise INH-resistant Mtb strains harboring mutations in the katG gene, which encodes the enzyme that converts the prodrug INH to its active form. Through mechanistic studies, we discovered that C10 inhibits Mtb respiration, revealing a link between respiration homeostasis and INH sensitivity. Therefore, by using C10 to dissect Mtb persistence, we discovered that INH resistance is not absolute and can be reversed.
Keywords: Mycobacterium tuberculosis; antibiotic resistance; drug tolerance; isoniazid; respiration.
Conflict of interest statement
Conflict of interest statement: C.L.S., S.J.H., and F.A. have ownership interests in Quretech Bio AB, which licenses C10.
Figures
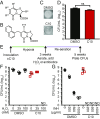

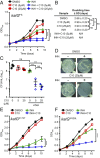
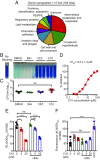


Comment in
-
Resistance Reversed in KatG Mutants of Mycobacterium tuberculosis.Trends Microbiol. 2019 Aug;27(8):655-656. doi: 10.1016/j.tim.2019.05.008. Epub 2019 Jun 6. Trends Microbiol. 2019. PMID: 31176512
References
-
- World Health Organization 2018 Global tuberculosis report. Available at https://apps.who.int/iris/bitstream/handle/10665/274453/9789241565646-en.... Accessed October 4, 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

