Properties of Aged Spores of Bacillus subtilis
- PMID: 31061168
- PMCID: PMC6597383
- DOI: 10.1128/JB.00231-19
Properties of Aged Spores of Bacillus subtilis
Abstract
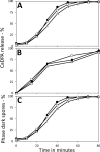
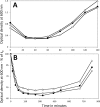
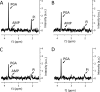
Bacillus spores incubated on plates for 2 to 98 days at 37°C had identical Ca-dipicolinic acid contents, exhibited identical viability on rich- or poor-medium plates, germinated identically in liquid with all germinants tested, identically returned to vegetative growth in rich or minimal medium, and exhibited essentially identical resistance to dry heat and similar resistance to UV radiation. However, the oldest spores had a lower core water content and significantly higher wet heat and NaOCl resistance. In addition, 47- and 98-day spores had lost >98% of intact 16S and 23S rRNA and 97 to 99% of almost all mRNAs, although minimal amounts of mononucleotides were generated in 91 days. Levels of 3-phosphoglyceric acid (3PGA) also fell 30 to 60% in the oldest spores, but how the 3PGA was lost is not clear. These results indicate that (i) translation of dormant spore mRNA is not essential for completion of spore germination, nor is protein synthesis from any mRNA; (ii) in sporulation for up to 91 days at 37°C, the RNA broken down generates minimal levels of mononucleotides; and (iii) the lengths of time that spores are incubated in sporulation medium should be considered when determining conditions for spore inactivation by wet heat, in particular, in using spores to test for the efficacy of sterilization regimens.IMPORTANCE We show that spores incubated at 37°C on sporulation plates for up to 98 days have lost almost all mRNAs and rRNAs, yet the aged spores germinated and outgrew as well as 2-day spores, and all these spores had identical viability. Thus, it is unlikely that spore mRNA, rRNA, or protein synthesis is important in spore germination. Spores incubated for 47 to 98 days also had much higher wet heat resistance than 2-day spores, suggesting that spore "age" should be considered in generating spores for tests of sterilization assurance. These data are the first to show complete survival of hydrated spores for ∼100 days, complementing published data showing dry-spore survival for years.
Keywords: Bacillus; endospores; germination; heat resistance.
Copyright © 2019 American Society for Microbiology.
Figures






References
-
- Setlow P. 2016. Spore resistance properties, p 201–215. In Eichenberger P, Driks A (ed), The bacterial spore: from molecules to systems. ASM Press, Washington, DC.
-
- Setlow P, Johnson EA. 2012. Spores and their significance, p 45–79. In Doyle MP, Buchanan R (ed), Food microbiology, fundamentals and frontiers, 4th ed ASM Press, Washington, DC.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

