Raman spectrum spectral imaging revealing the molecular mechanism of Berberine-induced Jurkat cell apoptosis and the receptor-mediated Berberine delivery system
- PMID: 31061758
- PMCID: PMC6484975
- DOI: 10.1364/BOE.10.001581
Raman spectrum spectral imaging revealing the molecular mechanism of Berberine-induced Jurkat cell apoptosis and the receptor-mediated Berberine delivery system
Abstract
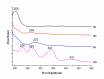
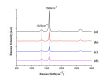
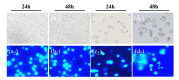
Berberine (BBR), a traditional Chinese herb extract medicine, reveals some anticancer effects in leukemia, but it remains controversial about the molecular mechanism of BBR-induced leukemia cell apoptosis. In this study, combining Raman spectrum and spectral imaging, both the biochemical changes of BBR-induced Jurkat cell apoptosis and the precise distribution of BBR in single cell are presented. In contrast, we also show the corresponding results of Jatrorrhizine (JTZ) and Palmatine (PMT), two structural analogues of BBR. It is found that all three structural analogues can induce cell apoptosis by breaking DNA and the main action sites are located in phosphate backbone and base pair groups, but their action on cell cycle are different, in which BBR leads to the S phase arrest while JTZ and PMT are on the G2 phase arrest. Moreover, from the Raman spectra of DNA treated with different drugs, we find that the content of phosphate backbone and base pair groups in BBR-treated DNA are larger than those in JTZ or PMT. And this result reflects the strong capability of BBR breaking DNA backbone relative to JTZ or PMT, suggesting that the existence of methylene-dioxy on the 2, 3 units of A ring on the quinoline ring can greatly enhance the capability of BBR breaking DNA backbone, so the action effect of BBR-induced Jurkat cell apoptosis is better than those of PMT or JTZ. Further, by using Raman spectral imaging approach, we achieve the precise distribution of BBR in single cell, it is found that the receptor-mediated BBR targeting delivery based single-wall carbon nanotube and folic acid (SWNT/FA) reveals excellent performance in BBR targeting delivery relative to the conventional BBR diffusion approach. Importantly, these results demonstrate that Raman spectrum and spectral imaging should be a powerful tool to study the molecular mechanism of drug-induced cell apoptosis and evaluate the efficiency of drug delivery system.
Conflict of interest statement
The authors declare that there are no conflicts of interest related to this article.
Figures













References
-
- Lu G. H., Wang L. M., Chen J. M., “Determination and utilization of berberine in plants of Berberis L,” J. Chin. Tradit. Herbal Drugs 30(6), 428–430 (1999).
-
- Xu S. H., He M. S., Liu X. P., “Berberine contents on B. triacanthophora,” J Chin Med Mater 26(3), 183–184 (2003).
-
- Wu H. L., Hsu C. Y., Liu W. H., Yung B. Y., “Berberine-induced apoptosis of human leukemia HL-60 cells is associated with down-regulation of nucleophosmin/B23 and telomerase activity,” Int. J. Cancer 81(6), 923–929 (1999). 10.1002/(SICI)1097-0215(19990611)81:6<923::AID-IJC14>3.0.CO;2-D - DOI - PubMed
LinkOut - more resources
Full Text Sources
