Perioperatively Inhaled Hydrogen Gas Diminishes Neurologic Injury Following Experimental Circulatory Arrest in Swine
- PMID: 31061920
- PMCID: PMC6488769
- DOI: 10.1016/j.jacbts.2018.11.006
Perioperatively Inhaled Hydrogen Gas Diminishes Neurologic Injury Following Experimental Circulatory Arrest in Swine
Abstract
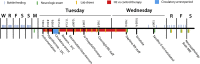
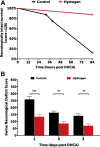
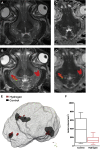
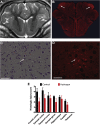
This study used a swine model of mildly hypothermic prolonged circulatory arrest and found that the addition of 2.4% inhaled hydrogen gas to inspiratory gases during and after the ischemic insult significantly decreased neurologic and renal injury compared with controls. With proper precautions, inhalational hydrogen may be administered safely through conventional ventilators and may represent a complementary therapy that can be easily incorporated into current workflows. In the future, inhaled hydrogen may diminish the sequelae of ischemia that occurs in congenital heart surgery, cardiac arrest, extracorporeal life-support events, acute myocardial infarction, stroke, and organ transplantation.
Keywords: CPB, cardiopulmonary bypass; GFAP, glial fibrillatory acidic protein; H2, hydrogen gas; PDI, Psychomotor Development Index; SNDS, Swine Neurodevelopment Score; circulatory arrest; hydrogen gas; ischemia-reperfusion injury; neuroprotection; •OH, hydroxyl radical.
Figures
References
-
- Algra S.O., Jansen N.J.G., van der Tweel I. Neurological injury after neonatal cardiac surgery: a randomized, controlled trial of 2 perfusion techniques. Circulation. 2014;129:224–233. - PubMed
-
- Dent C.L., Spaeth J.P., Jones B.V. Brain magnetic resonance imaging abnormalities after the Norwood procedure using regional cerebral perfusion. J Thorac Cardiovasc Surg. 2006;131:190–197. - PubMed
-
- Mahle W.T., Tavani F., Zimmerman R.A. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106:I109–I114. - PubMed
-
- McQuillen P.S., Barkovich A.J., Hamrick S.E.G. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. 2007;38:736–741. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

