Organ-sparing surgery of penile cancer: higher rate of local recurrence yet no impact on overall survival
- PMID: 31062123
- PMCID: PMC6994547
- DOI: 10.1007/s00345-019-02793-9
Organ-sparing surgery of penile cancer: higher rate of local recurrence yet no impact on overall survival
Abstract
Purpose: To report on the oncological outcome of organ-sparing surgery (OSS) compared to (total or partial) penectomy regarding recurrence patterns and survival in squamous cell carcinoma (SCC) of the penis.
Methods: This was a retrospective study of all patients with penile SCC and eligible follow-up data of at least 2 years at our institution. Patients with tumors staged ≥ pT1G2 underwent invasive lymph node (LN) staging by dynamic sentinel-node biopsy or modified inguinal lymphadenectomy. Radical inguinal lymphadenectomy was performed when LNs were palpable at diagnosis and in those with a positive LN status after invasive nodal staging. Follow-up visits were assessed, and local, regional and distant recurrences were defined and analyzed.
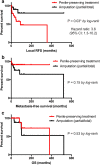
Results: 55 patients were identified with a mean follow-up of 63.7 months. Surgical management was OSS in 26 patients (47.2%) and partial or total penectomy in 29 cases (52.8%). Histopathological staging was: pTis (12.7%), pTa (16.3%), pT1a (18.2%), pT1b (5.5%), pT2 (29.1%) and pT3 (18.2%), respectively. Patients in the penectomy group were significantly older (mean 68 vs. 62 years; p = 0.026) with a higher rate of advanced tumor stage (≥ pT2: 44.8% vs. 11.5%; p = 0.002). The local recurrence rate was 42.3% (n = 11) following OSS compared to 10.3% (n = 3) after penectomy (p = 0.007). Kaplan-Meier curves showed no significant differences between the two groups regarding metastasis-free and overall survival.
Conclusions: OSS is associated with a higher local recurrence rate compared to penectomy, yet it has no negative impact on overall and metastasis-free survival.
Keywords: Follow-up; Organ-preserving surgery; Penile neoplasm; Recurrence; Squamous cell; Survival.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

