Evidence for altered excitatory and inhibitory tone in the post-mortem substantia nigra in schizophrenia
- PMID: 31062628
- PMCID: PMC6891153
- DOI: 10.1080/15622975.2019.1615638
Evidence for altered excitatory and inhibitory tone in the post-mortem substantia nigra in schizophrenia
Abstract
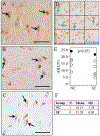
Objectives: The substantia nigra (SN) receives glutamatergic and GABAergic inputs that regulate dopaminergic neuronal activity. Imaging studies have shown hyperactivity of the SN in schizophrenia (SZ) patients. We examined neurochemically defined inputs to the SN, synaptic density, and neuromelanin content that might contribute to or reflect this hyperexcitability.Methods: Glutamatergic axon terminals were identified by the immunohistochemical localisation of vGLUT1 and vGLUT2; GABA inputs were identified by the immunohistochemical localisation of GAD67. Neuromelanin granules are visible in unstained sections and thus were assessed in unstained sections. Optical densitometry was measured to assess the density of vGLUT1, vGLUT2 or GAD67 immunolabelled axon terminals and neuromelanin granules. Electron microscopy was used to quantify synaptic and mitochondrial density.Results: Compared to controls, SZ subjects had nonsignificant trends toward a decrease in vGLUT1, and an increase in both vGLUT2 and GAD67. vGLUT1 was negatively correlated with GAD67 in normal controls (NCs) and positively correlated in SZ subjects. A correlation of coefficient analysis showed a significant difference between the negative correlation in NCs and the positive correlation in SZ subjects. Frequency histograms showed the distribution of neuromelanin density was different in SZ subjects compared to NCs. Synaptic density data showed a decrease in inhibitory synapses in SZ subjects. Mitochondrial density was normal in SZ subjects.Conclusions: Synaptic density alterations and the lack of a positive correlation between GAD67 and vGLUT1 could contribute to hyperactivity in the SN.
Keywords: Ultrastructure; dopamine; mitochondria; neuromelanin; synapses.
Figures







References
-
- Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Sampson AR, Lewis DA. 1999. Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. Am J Psychiatry 156(10):1580–1589. - PubMed
-
- Akil M, Edgar CL, Pierri JN, Casali S, Lewis DA. 2000. Decreased density of tyrosine hydroxylase immunoreactive axons in the entorhinal cortex of schizophrenic subjects. Biol Psychiatry 47:361–370. - PubMed
-
- American Psychiatric Association. 2013. Diagnostic and statistical manual of mental disorders (5th ed). Arlington, VA: American Psychiatric Publishing.
-
- Babcock DF, Hille B. 1998. Mitochondrial oversight of cellular Ca2+ signaling. Curr Opin Neurobiol 8:398–404. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
