The effectiveness of vaccination to prevent the papillomavirus infection: a systematic review and meta-analysis
- PMID: 31063090
- PMCID: PMC6518793
- DOI: 10.1017/S0950268818003679
The effectiveness of vaccination to prevent the papillomavirus infection: a systematic review and meta-analysis
Abstract
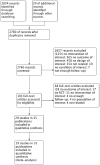
Our purpose was to determine the effectiveness and harms of vaccination in patients with any sexual history to prevent the prevalence of papillomavirus infection. A search strategy was conducted in the MEDLINE, CENTRAL, EMBASE and LILACS databases. Searches were also conducted in other databases and unpublished literature. The risk of bias was evaluated with the Cochrane Collaboration's tool. Analysis of fixed effects was conducted. The primary outcome was the infection by any and each human papillomavirus (HPV) genotype, serious adverse effects and short-term adverse effects. The measure of the effect was the risk difference (RD) with a 95% confidence interval (CI). The planned interventions were bivalent vaccine/tetravalent/nonavalent vs. placebo/no intervention/other vaccines. We included 29 studies described in 35 publications. Bivalent HPV vaccine offers protection against HPV16 (RD -0.05, 95% CI -0.098 to -0.0032), HPV18 (RD -0.03, 95% CI -0.062 to -0.0004) and HPV16/18 genotypes (RD of -0.1, 95% CI -0.16 to -0.04). On the other side, tetravalent HPV vaccine offered protection against HPV6 (RD of -0.0500, 95% CI -0.0963 to -0.0230), HPV11 (RD -0.0198, 95% CI -0.0310 to -0.0085). Also, against HPV16 (RD of -0.0608, 95% CI -0.1126 to -0.0091) and HPV18 (RD of -0.0200, 95% CI -0.0408 to -0.0123). There was a reduction in the prevalence of HPV16, 18 and 16/18 genotypes when applying the bivalent vaccine, with no increase in adverse effects. Regarding the tetravalent vaccine, we found a reduction in the prevalence of HPV6, 11, 16 and 18 genotypes, with no increase in adverse effects.
Keywords: Human papillomavirus; meta-analysis; systematic review; vaccine.
Figures
Similar articles
-
Effectiveness of bivalent and quadrivalent human papillomavirus vaccination in Luxembourg.Cancer Epidemiol. 2019 Dec;63:101593. doi: 10.1016/j.canep.2019.101593. Epub 2019 Sep 6. Cancer Epidemiol. 2019. PMID: 31499377
-
Prevalence of high-risk HPV and its genotypes-Implications in the choice of prophylactic HPV vaccine.J Med Virol. 2021 Aug;93(8):5188-5192. doi: 10.1002/jmv.27015. Epub 2021 Apr 23. J Med Virol. 2021. PMID: 33851736
-
Bivalent Vaccine Effectiveness Against Type-Specific HPV Positivity: Evidence for Cross-Protection Against Oncogenic Types Among Dutch STI Clinic Visitors.J Infect Dis. 2018 Jan 4;217(2):213-222. doi: 10.1093/infdis/jix582. J Infect Dis. 2018. PMID: 29140439 Free PMC article.
-
Immunogenicity and safety of human papillomavirus vaccine coadministered with other vaccines in individuals aged 9-25 years: A systematic review and meta-analysis.Vaccine. 2020 Jan 10;38(2):119-134. doi: 10.1016/j.vaccine.2019.10.092. Epub 2019 Dec 10. Vaccine. 2020. PMID: 31831220
-
Postvaccination prevalence of vaccine-Human Papillomavirus (vHPV) genotypes among the target population: A systematic review and meta-analysis.J Med Virol. 2021 Aug;93(8):4659-4667. doi: 10.1002/jmv.26968. Epub 2021 Apr 1. J Med Virol. 2021. PMID: 33764530
Cited by
-
Human Papillomavirus Vaccination and Physical and Mental Health Complaints Among Female Students in Secondary Education Institutions in Denmark.J Gen Intern Med. 2020 Sep;35(9):2647-2654. doi: 10.1007/s11606-020-05845-8. Epub 2020 Apr 27. J Gen Intern Med. 2020. PMID: 32342482 Free PMC article.
-
Strong reduction in prevalence of HPV16/18 and closely related HPV types in sexually active adolescent women following the introduction of HPV vaccination in Argentina.Papillomavirus Res. 2020 Dec;10:100208. doi: 10.1016/j.pvr.2020.100208. Epub 2020 Nov 6. Papillomavirus Res. 2020. PMID: 33161174 Free PMC article.
-
Human Papillomavirus (HPV) Infection and Risk Behavior in Vaccinated and Non-Vaccinated Paraguayan Young Women.Pathogens. 2024 Feb 27;13(3):209. doi: 10.3390/pathogens13030209. Pathogens. 2024. PMID: 38535552 Free PMC article.
-
Impact of industry sponsorship on the quality of systematic reviews of vaccines: a cross-sectional analysis of studies published from 2016 to 2019.Syst Rev. 2022 Aug 22;11(1):174. doi: 10.1186/s13643-022-02051-x. Syst Rev. 2022. PMID: 35996186 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous