Visualizing membrane trafficking through the electron microscope: cryo-tomography of coat complexes
- PMID: 31063149
- PMCID: PMC6503763
- DOI: 10.1107/S2059798319005011
Visualizing membrane trafficking through the electron microscope: cryo-tomography of coat complexes
Abstract
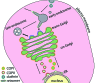
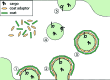
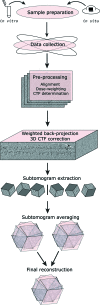
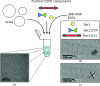
Coat proteins mediate vesicular transport between intracellular compartments, which is essential for the distribution of molecules within the eukaryotic cell. The global arrangement of coat proteins on the membrane is key to their function, and cryo-electron tomography and subtomogram averaging have been used to study membrane-bound coat proteins, providing crucial structural insight. This review outlines a workflow for the structural elucidation of coat proteins, incorporating recent developments in the collection and processing of cryo-electron tomography data. Recent work on coat protein I, coat protein II and retromer performed on in vitro reconstitutions or in situ is summarized. These studies have answered long-standing questions regarding the mechanisms of membrane binding, polymerization and assembly regulation of coat proteins.
Keywords: COPII; coat proteins; cryo-electron tomography; subtomogram averaging; three-dimensional reconstruction; vesicular transport.
open access.
Figures


References
-
- Antonny, B., Madden, D., Hamamoto, S., Orci, L. & Schekman, R. (2001). Nature Cell Biol. 3, 531–537. - PubMed
-
- Asano, S., Fukuda, Y., Beck, F., Aufderheide, A., Förster, F., Danev, R. & Baumeister, W. (2015). Science, 347, 439–442. - PubMed
-
- Barlowe, C., Orci, L., Yeung, T., Hosobuchi, M., Hamamoto, S., Salama, N., Rexach, M. F., Ravazzola, M., Amherdt, M. & Schekman, R. (1994). Cell, 77, 895–907. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

