OBG-like ATPase 1 inhibition attenuates angiotensin II-induced hypertrophic response in human ventricular myocytes via GSK-3beta/beta-catenin signalling
- PMID: 31063653
- PMCID: PMC6650359
- DOI: 10.1111/1440-1681.13101
OBG-like ATPase 1 inhibition attenuates angiotensin II-induced hypertrophic response in human ventricular myocytes via GSK-3beta/beta-catenin signalling
Abstract
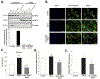
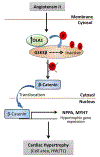
Obg-like ATPase 1 (OLA1) that possesses both GTP and ATP hydrolyzing activities has been shown to be involved in translational regulation of cancer cell growth and survival. Also, GSK3β signalling has been implicated in cardiac development and disease. However, the role of OLA1 in pathological cardiac hypertrophy is unknown. We sought to understand the mechanism by which OLA1 regulates GSK3β-β-Catenin signalling and its functional significance in angiotensin-II (ANG II)-induced cardiac hypertrophic response. OLA1 function and its endogenous interaction with GSK3β/β-catenin signalling in cultured human ventricular cardiomyocytes (AC16 cells) and mouse hearts (in vivo) was evaluated with/without ANG II-stimulated hypertrophic response. ANG II administration in mice increases myocardial OLA1 protein expression with a corresponding increase in GSK3β phosphorylation and decrease in β-Catenin phosphorylation. Cultured cardiomyocytes treated with ANG II show endogenous interaction between OLA1 and GSK3β, nuclear accumulation of β-Catenin and significant increase in cell size and expression of hypertrophic marker genes such as atrial natriuretic factor (ANF; NPPA) and β-myosin heavy chain (MYH7). Intriguingly, OLA1 inhibition attenuates the above hypertrophic response in cardiomyocytes. Taken together, our data suggest that OLA1 plays a detrimental role in hypertrophic response via GSK3β/β-catenin signalling. Translation strategies to target OLA1 might potentially limit the underlying molecular derangements leading to left ventricular dysfunction in patients with maladaptive cardiac hypertrophy.
Keywords: GSK3beta; OLA1; angiotensin II; beta-Catenin; cardiac hypertrophy.
© 2019 John Wiley & Sons Australia, Ltd.
Figures




References
-
- Ruilope LM, Schmieder RE: Left Ventricular Hypertrophy and Clinical Outcomes in Hypertensive Patients. Am J Hypertens 2008;21:500–508. - PubMed
-
- Sadoshima J, Izumo S: The Cellular and Molecular Response of Cardiac Myocytes to Mechanical Stress. Annu Rev Physiol 1997;59:551–571. - PubMed
-
- Frey N, Olson EN: Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol 2003;65:45–79. - PubMed
-
- Krishnamurthy P, Subramanian V, Singh M, Singh K: Beta1 integrins modulate beta-adrenergic receptor-stimulated cardiac myocyte apoptosis and myocardial remodeling. Hypertens Dallas Tex 1979 2007;49:865–872. - PubMed
-
- Aikawa R, Komuro I, Nagai R, Yazaki Y: Rho plays an important role in angiotensin II-induced hypertrophic responses in cardiac myocytes. Mol Cell Biochem 2000;212:177–182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

