Systematic Review of Patient-Derived Xenograft Models for Preclinical Studies of Anti-Cancer Drugs in Solid Tumors
- PMID: 31064068
- PMCID: PMC6562882
- DOI: 10.3390/cells8050418
Systematic Review of Patient-Derived Xenograft Models for Preclinical Studies of Anti-Cancer Drugs in Solid Tumors
Abstract
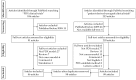
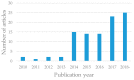
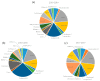
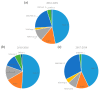
Patient-derived xenograft (PDX) models are used as powerful tools for understanding cancer biology in PDX clinical trials and co-clinical trials. In this systematic review, we focus on PDX clinical trials or co-clinical trials for drug development in solid tumors and summarize the utility of PDX models in the development of anti-cancer drugs, as well as the challenges involved in this approach, following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines. Recently, the assessment of drug efficacy by PDX clinical and co-clinical trials has become an important method. PDX clinical trials can be used for the development of anti-cancer drugs before clinical trials, with their efficacy assessed by the modified response evaluation criteria in solid tumors (mRECIST). A few dozen cases of PDX models have completed enrollment, and the efficacy of the drugs is assessed by 1 × 1 × 1 or 3 × 1 × 1 approaches in the PDX clinical trials. Furthermore, co-clinical trials can be used for personalized care or precision medicine with the evaluation of a new drug or a novel combination. Several PDX models from patients in clinical trials have been used to assess the efficacy of individual drugs or drug combinations in co-clinical trials.
Keywords: PDX clinical trial; co-clinical trial; drug development; drug sensitivity; patient-derived cancer model; solid tumor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Cunningham D., Humblet Y., Siena S., Khayat D., Bleiberg H., Santoro A., Bets D., Mueser M., Harstrick A., Verslype C., et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. - DOI - PubMed
-
- Smith I., Procter M., Gelber R.D., Guillaume S., Feyereislova A., Dowsett M., Goldhirsch A., Untch M., Mariani G., Baselga J., et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: A randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

