Presynaptic Calcium Channels
- PMID: 31064106
- PMCID: PMC6539076
- DOI: 10.3390/ijms20092217
Presynaptic Calcium Channels
Abstract
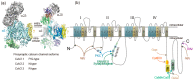
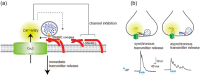
Presynaptic Ca2+ entry occurs through voltage-gated Ca2+ (CaV) channels which are activated by membrane depolarization. Depolarization accompanies neuronal firing and elevation of Ca2+ triggers neurotransmitter release from synaptic vesicles. For synchronization of efficient neurotransmitter release, synaptic vesicles are targeted by presynaptic Ca2+ channels forming a large signaling complex in the active zone. The presynaptic CaV2 channel gene family (comprising CaV2.1, CaV2.2, and CaV2.3 isoforms) encode the pore-forming α1 subunit. The cytoplasmic regions are responsible for channel modulation by interacting with regulatory proteins. This article overviews modulation of the activity of CaV2.1 and CaV2.2 channels in the control of synaptic strength and presynaptic plasticity.
Keywords: Ca2+ binding proteins; Ca2+ channels; G-proteins; synaptic proteins; synaptic transmission.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

