HER2 Upregulates ATF4 to Promote Cell Migration via Activation of ZEB1 and Downregulation of E-Cadherin
- PMID: 31064130
- PMCID: PMC6540102
- DOI: 10.3390/ijms20092223
HER2 Upregulates ATF4 to Promote Cell Migration via Activation of ZEB1 and Downregulation of E-Cadherin
Abstract
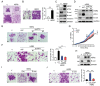
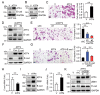
HER2 (human epidermal growth factor receptor 2) activation is critical in breast cancer development. HER2 promotes cell proliferation, angiogenesis, survival, and metastasis by activation of PI3K/Akt, Ras/MEK/ERK, and JAK/STAT pathways. However, beyond these signaling molecules, the key proteins underlining HER2-mediated metastasis remain elusive. ATF4 (Activating transcription factor 4), a critical regulator in unfolded protein response (UPR), is implicated in cell migration and tumor metastasis. In this study, we demonstrate that HER2 upregulated ATF4 expression at both mRNA and protein levels, resulting in cell migration increased. In addition, ATF4 upregulated ZEB1 (Zinc finger E-box-binding homeobox 1) and suppressed E-cadherin expression resulting in promoting cell migration. Restoration of E-cadherin expression effectively inhibited HER2- or ATF4-mediated cell migration. In addition, upregulated expression of ATF4 was found in HER2-positive breast cancer specimens. Together, this study demonstrates that ATF4-ZEB1 is important for HER2-mediated cell migration and suggests that ATF4-ZEB1 may be potential therapeutic targets for breast cancer metastasis.
Keywords: ATF4; E-cadherin; HER2; ZEB1; cell migration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Verri E., Guglielmini P., Puntoni M., Perdelli L., Papadia A., Lorenzi P., Rubagotti A., Ragni N., Boccardo F. Her2/neu oncoprotein overexpression in epithelial ovarian cancer: Evaluation of its prevalence and prognostic significance. Clinical study. Oncology. 2005;68:154–161. doi: 10.1159/000086958. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

