Establishment of a rat ovarian peritoneal metastasis model to study pressurized intraperitoneal aerosol chemotherapy (PIPAC)
- PMID: 31064330
- PMCID: PMC6503553
- DOI: 10.1186/s12885-019-5658-5
Establishment of a rat ovarian peritoneal metastasis model to study pressurized intraperitoneal aerosol chemotherapy (PIPAC)
Abstract
Background: pressurized intraperitoneal aerosol chemotherapy (PIPAC), with or without electrostatic precipitation (ePIPAC), was recently introduced in the treatment of peritoneal metastases (PM) from ovarian cancer (OC). Preliminary clinical data are promising, but several methodological issues as well the anticancer efficacy of PIPAC remain unaddressed. Here, we propose a rat ePIPAC model that allows to study these issues in a clinically relevant, reproducible, and high throughput model.
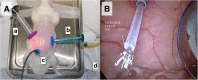
Methods: laparoscopy and PIPAC were established in healthy Wistar rats. Aerosol properties were measured using laser diffraction spectrometry based granulometric analyses. Electrostatic precipitation was accomplished using a commercially available generator (Ultravision™). A xenograft model of ovarian PM was created in athymic rats using intraperitoneal (IP) injection of SKOV-3 luciferase positive cells. Tumor growth was monitored weekly by in vivo bioluminescence imaging.
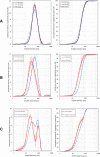
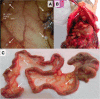
Results: PIPAC and electrostatic precipitation were well tolerated using a capnoperitoneum of 8 mmHg. All rats survived the (e)PIPAC procedure and no gas or aerosol leakage was observed over the entire procedure. With an injection pressure of 20 bar, granulometry showed a mean droplet diameter (D(v,0.5)) of 47 μm with a flow rate of 0.5 mL/s, and a significantly lower diameter (30 μm) when a flow rate of 0.8 mL/s was used. Experiments using IP injection of SKOV-3 luciferase positive cells showed that after IP injection of 20 × 106 cells, miliary PM was observed in all animals. PIPAC was feasible and well supported in these tumor bearing animals.
Conclusions: we propose a reproducible and efficient rodent model to study PIPAC and ePIPAC in OC xenografts with widespread PM. This model allows to characterize and optimize pharmacokinetic and biophysical parameters, and to evaluate the anti-cancer efficacy of (e)PIPAC treatment.
Keywords: Intraperitoneal drug delivery; Laparoscopic surgery; Ovarian cancer; PIPAC; Peritoneal metastases; Rat xenograft; ePIPAC.
Conflict of interest statement
Ethics approval
The study protocol was approved by the Animal Ethical Committee of the Faculty of Medicine, Ghent University, Belgium (ECD 17–50 and ECD 18–30). The human cell lines did not require ethics approval from our institution as they were purchased from legal commercial product.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Oseledchyk A, Zivanovic O. Intraoperative Hyperthermic intraperitoneal chemotherapy in patients with advanced ovarian Cancer. Oncology (Williston Park, NY) 2015;29:695–701. - PubMed
-
- Klaver YLB, Simkens LHJ, Lemmens VEPP, Koopman M, Teerenstra S, Bleichrodt RP, de Hingh IHJT, Punt CJA. Outcomes of colorectal cancer patients with peritoneal carcinomatosis treated with chemotherapy with and without targeted therapy. Eur J Surg Oncol. 2012;38:617–623. doi: 10.1016/j.ejso.2012.03.008. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

