High-level extracellular production of recombinant nattokinase in Bacillus subtilis WB800 by multiple tandem promoters
- PMID: 31064343
- PMCID: PMC6505213
- DOI: 10.1186/s12866-019-1461-3
High-level extracellular production of recombinant nattokinase in Bacillus subtilis WB800 by multiple tandem promoters
Abstract
Background: Nattokinase (NK), which is a member of the subtilisin family, is a potent fibrinolytic enzyme that might be useful for thrombosis therapy. Extensive work has been done to improve its production for the food industry. The aim of our study was to enhance NK production by tandem promoters in Bacillus subtilis WB800.
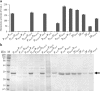
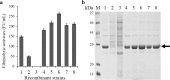
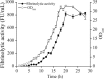
Results: Six recombinant strains harboring different plasmids with a single promoter (PP43, PHpaII, PBcaprE, PgsiB, PyxiE or PluxS) were constructed, and the analysis of the fibrinolytic activity showed that PP43 and PHpaII exhibited a higher expression activity than that of the others. The NK yield that was mediated by PP43 and PHpaII reached 140.5 ± 3.9 FU/ml and 110.8 ± 3.6 FU/ml, respectively. These promoters were arranged in tandem to enhance the expression level of NK, and our results indicated that the arrangement of promoters in tandem has intrinsic effects on the NK expression level. As the number of repetitive PP43 or PHpaII increased, the expression level of NK was enhanced up to the triple-promoter, but did not increase unconditionally. In addition, the repetitive core region of PP43 or PHpaII could effectively enhance NK production. Eight triple-promoters with PP43 and PHpaII in different orders were constructed, and the highest yield of NK finally reached 264.2 ± 7.0 FU/ml, which was mediated by the promoter PHpaII-PHpaII-PP43. The scale-up production of NK that was promoted by PHpaII-PHpaII-PP43 was also carried out in a 5-L fermenter, and the NK activity reached 816.7 ± 30.0 FU/mL.
Conclusions: Our studies demonstrated that NK was efficiently overproduced by tandem promoters in Bacillus subtilis. The highest fibrinolytic activity was promoted by PHpaII-PHpaII-PP43, which was much higher than that had been reported in previous studies. These multiple tandem promoters were used successfully to control NK expression and might be useful for improving the expression level of the other genes.
Keywords: Bacillus subtilis; Core promoter region; Nattokinase; Recombinant enzyme; Tandem promoter.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Fujita M, Nomura K, Hong K, Ito Y, Asada A, Nishimuro S. Purification and characterization of a strong fibrinolytic enzyme (Nattokinase) in the vegetable cheese Natto, a popular soybean fermented food in Japan. Biochem Bioph Res Commun. 1993;197(3):1340–1347. doi: 10.1006/bbrc.1993.2624. - DOI - PubMed
-
- Urano T, Ihara H, Umemura K, Suzuki Y, Oike M, Akita S, Tsukamoto Y, Suzuki I, Takada A. The profibrinolytic enzyme subtilisin NAT purified from Bacillus subtilis cleaves and inactivates plasminogen activator inhibitor type 1. J Biol Chem. 2001;276(27):24690–24696. doi: 10.1074/jbc.M101751200. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

