Hyperfiltration predicts long-term renal outcomes in humanized sickle cell mice
- PMID: 31064747
- PMCID: PMC6517665
- DOI: 10.1182/bloodadvances.2018028878
Hyperfiltration predicts long-term renal outcomes in humanized sickle cell mice
Abstract
We previously reported that humanized sickle cell (HbSS) mice develop spontaneous nephropathy, a major cause of morbidity and mortality in sickle cell disease (SCD). Because sex-dependent protective mechanisms in SCD have been reported, we examined the course of nephropathy in male and female HbSS mice to determine contributors and/or predictors of disease severity. In male HbSS mice, glomerular filtration rate was characterized by a rapid onset of hyperfiltration and subsequent progressive decline of renal function over 20 weeks. Early tubular injury presented with increased excretion of kidney injury marker 1 (KIM-1), progressive loss of tubular brush border, and interstitial fibrosis that preceded the onset of glomerular damage, suggesting a tubuloglomerular mechanism of kidney injury in these mice. Additionally, we observed a strong association between the magnitude of hyperfiltration and the degree of long-term kidney injury in male HbSS mice. Unlike males, female HbSS mice did not demonstrate a significant loss of renal function or severe kidney damage during the time course of the study. These results suggest that magnitude of hyperfiltration predicts the onset of chronic kidney damage in male HbSS mice, whereas protective mechanisms in female HbSS mice delay the onset of SCD nephropathy.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
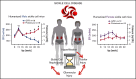
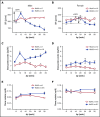
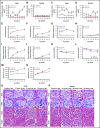
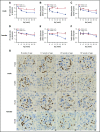
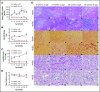



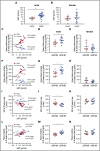
Comment in
-
Sex differences in the trajectory of glomerular filtration rate in pediatric and murine sickle cell anemia.Blood Adv. 2020 Jan 28;4(2):263-265. doi: 10.1182/bloodadvances.2019001237. Blood Adv. 2020. PMID: 31951651 Free PMC article. No abstract available.
References
-
- Guasch A, Navarrete J, Nass K, Zayas CF. Glomerular involvement in adults with sickle cell hemoglobinopathies: Prevalence and clinical correlates of progressive renal failure. J Am Soc Nephrol. 2006;17(8):2228-2235. - PubMed
-
- Nielsen L, Canouï-Poitrine F, Jais JP, et al. . Morbidity and mortality of sickle cell disease patients starting intermittent haemodialysis: a comparative cohort study with non- Sickle dialysis patients. Br J Haematol. 2016;174(1):148-152. - PubMed
-
- Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. . Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312(10):1033-1048. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

