Oxidative and endoplasmic reticulum stresses are involved in palmitic acid-induced H9c2 cell apoptosis
- PMID: 31064816
- PMCID: PMC6527925
- DOI: 10.1042/BSR20190225
Oxidative and endoplasmic reticulum stresses are involved in palmitic acid-induced H9c2 cell apoptosis
Abstract
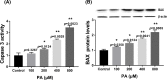
Palmitic acid (PA) is the most common saturated long-chain fatty acid that causes damage to heart muscle cells. However, the molecular mechanism of PA toxicity in myocardial cells is not fully understood. In the present study, we explored the effects of PA on proliferation and apoptosis of H9c2 cardiomyocytes, and uncovered the signaling pathways involved in PA toxicity. Our study revealed induction of both oxidative and endoplasmic reticulum (ER) stresses and exacerbation of apoptosis in PA-treated H9c2 cells. Inhibition of oxidative stress by N-acetylcysteine (NAC) reduced apoptosis and decreased ER stress in PA-treated H9c2 cells. Moreover, inhibition of ER stress by 4-phenyl butyric acid decreased apoptosis and attenuated oxidative stress. In summary, the present study demonstrated that oxidative stress coordinates with ER stress to play important roles in PA-induced H9c2 cell apoptosis.
Keywords: H9c2 cells; apoptosis; endoplasmic reticulum stress; oxidative stress; palmitic acid.
© 2019 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
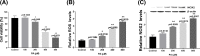



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

