Purinergic Signaling Pathway in Human Olfactory Neuronal Precursor Cells
- PMID: 31065271
- PMCID: PMC6466875
- DOI: 10.1155/2019/2728786
Purinergic Signaling Pathway in Human Olfactory Neuronal Precursor Cells
Abstract
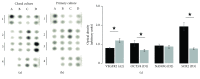
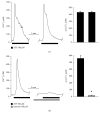
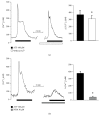
Extracellular ATP and trophic factors released by exocytosis modulate in vivo proliferation, migration, and differentiation in multipotent stem cells (MpSC); however, the purinoceptors mediating this signaling remain uncharacterized in stem cells derived from the human olfactory epithelium (hOE). Our aim was to determine the purinergic pathway in isolated human olfactory neuronal precursor cells (hONPC) that exhibit MpSC features. Cloning by limiting dilution from a hOE heterogeneous primary culture was performed to obtain a culture predominantly constituted by hONPC. Effectiveness of cloning to isolate MpSC-like precursors was corroborated through immunodetection of specific protein markers and by functional criteria such as self-renewal, proliferation capability, and excitability of differentiated progeny. P2 receptor expression in hONPC was determined by Western blot, and the role of these purinoceptors in the ATP-induced exocytosis and changes in cytosolic Ca2+ ([Ca2+]i) were evaluated using the fluorescent indicators FM1-43 and Fura-2 AM, respectively. The clonal culture was enriched with SOX2 and OCT3/4 transcription factors; additionally, the proportion of nestin-immunopositive cells, the proliferation capability, and functionality of differentiated progeny remained unaltered through the long-term clonal culture. hONPC expressed P2X receptor subtypes 1, 3-5, and 7, as well as P2Y2, 4, 6, and 11; ATP induced both exocytosis and a transient [Ca2+]i increase predominantly by activation of metabotropic P2Y receptors. Results demonstrated for the first time that ex vivo-expressed functional P2 receptors in MpSC-like hONPC regulate exocytosis and Ca2+ signaling. This purinergic-triggered release of biochemical messengers to the extracellular milieu might be involved in the paracrine signaling among hOE cells.
Figures






References
LinkOut - more resources
Full Text Sources
Miscellaneous

